eISSN: 2576-4543


Short Communication Volume 1 Issue 4
Department of Chemical Engineering, SV National Institute of Technology, India
Correspondence: Jignasa V Gohel, Department of Chemical Engineering, SV National Institute of Technology, Surat-395007, Gujarat, India, Tel (0261) 2201642
Received: August 21, 2017 | Published: November 9, 2017
Citation: Patel SB, Gohel JV. Effect of type of solvent on the sol-Gel spin coated CZTS thin films. Phys Astron Int J. 2017;1(4):126-129. DOI: 10.15406/paij.2017.01.00023
In recent times, the kieserite material based thin film solar cells are acquiring a substantial attention. The quaternary Cu2ZnSnS4 (CZTS) is considered as promising next generation semiconducting material owing to requirement earth abundant and environmentally constituents, its direct band gap of 1.4-1.6eV with p-type conductivity and high absorbance coefficient (104 cm-1). In this paper, the effect of type of solvent on the sol-gel spin coated CZTS thin film is studied. The sol-gel solution is prepared using four different environment-friendly solvents, namely, Dimethyl sulfoxide, Isopropyl alcohol, Ethylene glycol and Propylene glycol. The thin film prepared using the most cost effective spin coating technique. XRD patterns of all the prepared samples shows single phase CZTS with preferential orientation along (112) direction for kieserite structure. Morphological analysis is carried out using SEM. The morphological analysis suggested the sample prepared using propylene glycol is superior. The optical analysis is carried out using UV-Visible and it also suggested that propylene glycol is best suitable solvent as best band gap (1.51eV) is achieved. The solar cell is fabricated for CZTS thin film prepared using propylene glycol and 3.02% of solar cell efficiency is achieved.
Keywords: CZTS, thin film, Sol-gel spin coating, solvent
Kesterite material based thin film solar cells are most favorable to replace currently available silicon based solar cell owing to low fabrication cost and good stability. At present, copper indium gallium selenide (CIGS) and cadmium telluride (CdTe) based solar cells are available in the market. However, due to the toxicity of cadmium and selenide and less availability of indium and tellurium in earth crush, the large scale production of these material based solar cell is limited.1 Hence, the new emerging kieserite quaternary material copper zinc tin sulfide (CZTS) is considered as next generation thin film absorber material.2 CZTS requires easily available in earth crush and eco-friendly materials. CZTS also possess a good direct band gap of 1.4-1.6eV which is close to theoretically reported optimum band gap (1.45eV), high absorbance coefficient (104cm-1) with p-type conductivity.3
Up till now, numerous CZTS thin film preparation methods are reported, such as electrode position,4 pulse laser deposition,5 thermal deposition,6 spray pyrolysis,7 sol-gel spin coating,8 etc. Among these techniques, the sol-gel spin coating is most preferred for low-cost thin film preparation.9 CZTS is a quaternary substance, hence it is very difficult to avoid the secondary phase generation. To obtain high-efficiency devices, the control over secondary phase formation in CZTS is crucial. It requires good control over process parameters. In recent years, there is significant work has been carried out to improve sol-gel spin coated CZTS thin film optical and electrical properties. However still, an extensive research is required as highest reported around 7%, which is lower than theoretically calculated (32%).10 In our previous study,11 the CZTS thin film quality controlling parameters are listed. Selection of solvent is one of the key parameters for sol-gel spin coating. Solvent controls optical, structural as well as morphological properties of the thin film in the sol-gel spin coating. Hence, in the present study, the effect of type of solvent on the properties of CZTS thin film is studied extensively. Further, the solar cell is fabricated and efficiency is measured.
The four different solution was prepared using four different environment-friendly solvents, namely, DMSO, IPA, EG, and PG. Copper chloride (CuCl2.2H2O), zinc acetate (Zn(O2CCH3)2(H2O)2), tin chloride (SnCl2.2H2O) and thiourea (SC(NH2)2) were used as a source of Cu, Zn, Sn, and S, respectively. The precursors were added into DMSO and stirred for 60 min at 50°C temperature. A few drops of MEA were added as a stabilizer. A clear yellowish solution is acquired after completion of the process. The same procedure was carried out for IPA, EG, and PG and a clear yellow solution was obtained. Further, the solution was applied on the FTO glass substrate and coated at 2000rpm. Subsequently, the film is baked at 230°C. The coating process was repeated for 7-8 times and finally, the film is annealed under vacuum at 500°C, to avoid toxic H2S annealing. Four different films were prepared using different solvents based solutions.
The structural property of CZTS film is characterized by XRD using Rigaku D/Max 2200 system with CuKα radiation (CuKα=1.54Å). The XRD pattern suggested that all the films are single phase CZTS thin film (see Figure 1). No evidence on secondary phase is observed. All the films have exhibited (112) plane orientation which leads to the presence of frequently reported kieserite structure.12–15 The superior intensity is achieved by film prepared using PG as a solvent, which suggests the better crystalline is achieved.
The surface morphology of CZTS thin film is studied using scanning electron microscope S3400, Hitachi International Ltd. The surface morphology is hugely affected by solvent variation. The different solvent formed different shaped and sized nano particles Figure 2(a-d). Figure 2a depicts the surface morphology of thin film prepared using DMSO as a solvent. A clearly rope shaped surface morphology with minor voids can be observed for DMSO. The thin film prepared using IPA as solvent exhibited connected nano particle as elements are connected in DNA (Figure 2b). However, some voids can be observed. The film prepared using EG as solvent shows flower shape morphology (Figure 2c). However, agglomeration of nano particles can be observed as well. It is well reported that the uniform surface morphology is essential, as agglomeration may lead to the reduction in grain boundaries.9,16–18 It leads to increase in the effective diffusion length of minority carrier, which ultimately leads to poor solar cell performance.19 Ali et al.20 have reported that voids can adversely affect the electrical and optical properties of the films. Hence, both agglomeration and voids are not desired. A well dispersed, uniform and dense surface morphology is observed for the thin film prepared using PG as solvent (Figure 2d). Hence, the film prepared using PG as a solvent is considered the best.
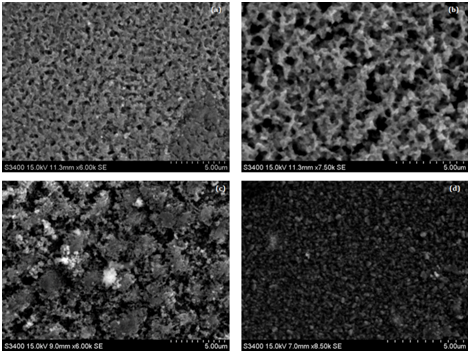
Figure 2 Surface morphology of thin film prepared by using (a) DMSO (b) IPA (c) EG and (d) PG as solvent.
Optical analysis of the thin film is carried out using HACH-DR 6000/2 UV spectrometer. The band gap is evaluated using Tauc’s plot method. Figure 3 illustrates the Tauc’s plot of thin film prepared using different solvents. However, the solvent variation doesn’t affect much the optical properties of thin films as all films exhibited band gap in the range of 1.5-1.6eV, which is frequently reported.21–23Shockley and Queisser had theoretically calculated that maximum solar cell efficiency (33%) can be achieved at low band gap, preferably at 1.45eV.24 Hence, preferably low band gap is desired for the superior solar cell performance.
However, in the present study, the film preparation with another solvent could not lead to low band gap. The films prepared with DMSO IPA and EG led to high band gap of 1.6eV, 1.55eV 1.53eV, respectively. The minimum band gap is only achieved with the film prepared with PG as a solvent; hence, it may be termed as superior film compared other films. Basically, the superior crystalline and surface morphology is achieved for the film prepared with PG. In addition, the superior crystalline and surface morphology can further lead to large grain size. And it is well reported that larger the grain size, smaller is the band gap.23 Hence, attributed to the superior crystalline and surface morphology achieved with the film prepared by PG, the film depicted smaller and superior band gap, in the present study.
The solar cell is fabricated by the thin film prepared using PG as a solvent. The frequently reported CZTS solar cell structure is TCO/CZTS/CdS/ZnO/Back contact.25–27 In the present study, FTO is used as TCO and CuS/FTO is used as a back contact to comply the tandem cell principle. Hence, cell structure becomes FTO/CZTS/CdS/ZnO/CuS/FTO. The solar cell performance is measured using Keithley 2400 under AM 1.5G illumination at 1000W/m2. The solar cell has exhibited 3.02% of PCE with 15.6 mAcm-2 current density (JSC), 450mV open circuit voltage (VOC) and 43% fill factor. Figure 4 depicts the J-V characteristics of the solar cell.
In the present study, the effect of type of solvent on the optical and electrical properties of CZTS thin film is studied extensively. The four environ-friendly solvents, namely, DMSO, IPA, EG, and PG are used for the preparation of the sol-gel solution. The CZTS thin film is prepared successfully using spin coating technique. All four thin film exhibited kieserite structure. However, the superior intensity is achieved by film prepared using PG as a solvent. The solvent affected surface morphology of thin film extensively. Different solvent formed different shaped and size nano particles. DMSO formed rope shaped morphology with minor voids, IPA formed DNA type connected surface morphology with voids, EG formed flower shaped morphology with some agglomeration of nano particles. Uniform, well dispersed and dense morphology is achieved by film prepared using PG as a solvent. Optical properties are not affected widely by the solvent variation. However, superior band gap (1.51eV) is achieved by film prepared using PG as a solvent. A tandem cell is fabricated by thin film prepared using PG as a solvent and 3.02% of PCE is achieved.
The authors would like to acknowledge the sophisticated instrumentation center and Department of Chemical Engineering of S.V. National Institute of Technology, Surat, India.
Author Declare there is no conflict of interest.

©2017 Patel, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.