
Mini Review Volume 1 Issue 2
A brief overview about laser-ablated molybdenum plasma properties
Sternberg EMA,1
Regret for the inconvenience: we are taking measures to prevent fraudulent form submissions by extractors and page crawlers. Please type the correct Captcha word to see email ID.

Rodrigues NAS2
1Department of Physics, Federal Institute of Education, Brazil
2Department of Physics, Aeronautics Institute of Technology, Brazil
Correspondence: Emmanuela Sternberg, Department of Physics, Federal Institute of Education, Science and Technology of Esp, Tel (+55) 27 32461600
Received: August 16, 2017 | Published: September 8, 2017
Citation: Sternberg EMA, Rodrigues NAS. A brief overview about laser-ablated molybdenum plasma properties. Phys Astron Int J. 2017;1(2):49-52. DOI: 10.15406/paij.2017.01.00008
Download PDF
Abstract
First step in molybdenum isotope separation for medical purposes project, based in laser ablation followed by electromagnetic separation methods, is the characterization of ablated plasma plume. In this work, a depth profile emission spectroscopy study of laser-ablated molybdenum plasma expanding on air at atmospheric pressure was performed, considering Local Thermodynamic Equilibrium and one-dimensional uniform expanding models. Plasma temperature, electron density, electron population distribution and electron impact width parameter were evaluated along plasma expansion axis and reach average values of 15000 K, 1018 cm-3, 1% of electron population at 4d45s6s state and (0.017±0.008) nm, respectively. Furthermore, mean velocity of (5.0±0.7) km/s and the plasma lifetime that was (160 ± 14) ns for length equal to (1.80±0.05) mm were measured. So, this work includes a description of mainly plasma plume parameters, which permits a discussion for isotope separation methods related to laser ablation.
Keywords: laser, plasma plum, wavelength, electronic population, atmospheric pressure, atoms
Introduction
In Nuclear Medicine, the 99m technetium isotope, 99mTc, is used as tracer in CT diagnosis exams, and the only source of 99mTc is beta-decay of 99 molybdenum isotope, 99Mo. The problem is that a few aging reactors are working for attending world’s needs of 99Mo, besides the fact that these processes involve highly enriched uranium (HEU).1–3
Intending to solve this problem, new methods for 99Mo production are being proposed, such as neutron activation of 98Mo. To improve this method, it is suggested isotope separation methods to enhance the abundance of 99Mo on the sample. A preliminary step to implement the suggested method is the generation and characterization of a laser-ablated plasma plume. Laser induced breakdown spectroscopy of molybdenum can determine expansion speed and lifetime4 after laser pulse of plasma jet, the portion of neutral and ionized species which constitutes the plasma, electronic population distribution,5 dynamics of plume expansion,4 electron density, temperature and electron impact width parameter.6
This work consists on laser ablation of molybdenum solid target and the characterization of the resulting plasma plume, aiming to analyze the possibility of application of isotope separation methods. So, the characterization of molybdenum by laser induced breakdown spectroscopy contributes not only for 99Mo production but also with understanding fundamental concepts on laser-ablated plasmas.
Experimental setup
For molybdenum plasma plume generation, a Coherent AVIA 355-X Nd:YAG laser emitting pulses with 0.205 mJ energy and 25 ns duration (FWHM) in third harmonic (355 nm) was focused on a molybdenum solid sample with a laser fluence of 6 J/cm2 and repetition rate equal to 1.2 kHz. The solid sample was placed on a XY table, which movement is controlled by EMC2 software, intending to avoid sample cratering and plasma extinguishing. Figure 1 indicates a schematic experimental setup diagram.
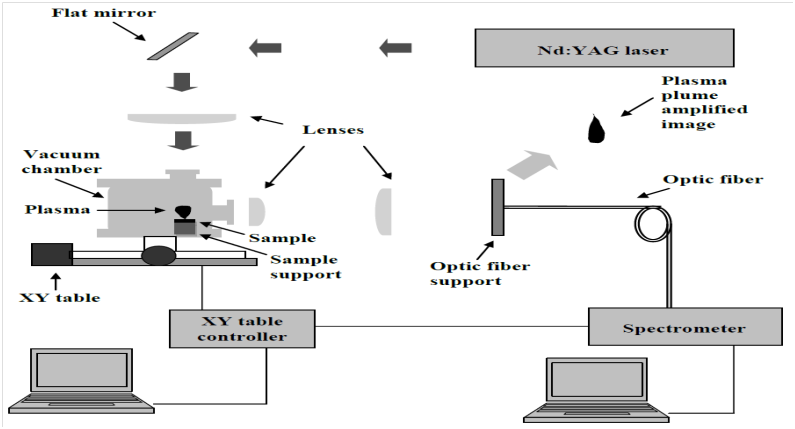
Figure 1 Experimental set-up.4
The plasma emitted light was collected by lenses set and focused on an optical fiber supported on a XYZ micrometer, which permits to execute a space-resolved study of plasma light distribution. Emitted light was analyzed through HR4000 and LIBS 2500+ spectrometers, both from Ocean Optics.
Results and discussions
The acquired spectra from plasma emitted light were investigated through direct comparison with theoretical spectra (Figure 2) constructed from NIST database for molybdenum neutral and singly ionized emission lines.7 Based on assignment of emission lines for all emission wavelength range, it can be concluded that the light emitted from the plasma near the surface of the target is, mainly, due de-excitation of molybdenum ionized atoms and, as the plasma expands, it is due to transitions of neutral atoms to the ground state.

Figure 2 Assignment of the molybdenum emission lines for neutral atoms (Mo I).
Dashed line represents experimental data and solid line is theoretical spectrum.4
Assigned experimental lines were used to determine electron temperature, electron density and electron population distribution, considering Local Thermodynamic Equilibrium Model. In this case, Saha equation (Equation 1) relates the electron density of the states with electron temperature for different ionization stages.9
(1)
In which
is electron density of the state,
is degeneracy,
is electron mass,
is energy of the level,
is ionization energy. It is possible to establish a connection of emission line intensity and its electronic transition, so Equation 2 relates intensity
and wavelengthλof emission line with electron temperature and total electron density, where A is spontaneous emission coefficient (and can be related to the probability of spontaneous emission),
is Boltzmann´s constant,
is Planck´s constant, and sub-indexes +, 0 and
are related to the ionized atoms, neutral atoms and electrons, respectively.
(2)
Electron temperature along plasma expansion axis was determined through Boltzmann plot (or its simplification called two lines method), when only information of emission lines related to transitions of atoms at the same ionization degree, and Saha-Boltzmann plot for the ratio of emission lines from different ionization degrees. So, electron temperature is inversely proportional to angular coefficient of linear fitting. Fig. 3 presents a typical behavior of electron temperature, with a maximum value at the plume core, with mean value nearly 15000 K.
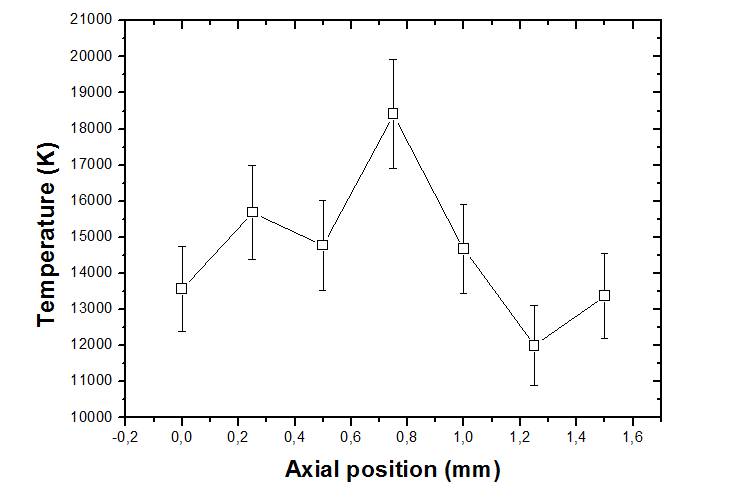
Figure 3 Electron temperature evolution along plume expansion axis.5
Replacing the calculated values for electron temperature in Equation 2, electron density
was direct determined. A representative behavior of electron density along plasma expansion axis is depicted in Figure 4a and permits to evaluate that, at central portion of the plume, electron density reaches values around 1018 particles/cm3. Once electron temperature values were measured, it also can be estimated a proportion of electron distribution population of each electronic level by means of Saha equation (Equation 1). Figure 4b reveals the time evolution of electron population of 4d45s6s state, where, at the second moment, only spontaneous emission was taken into account.
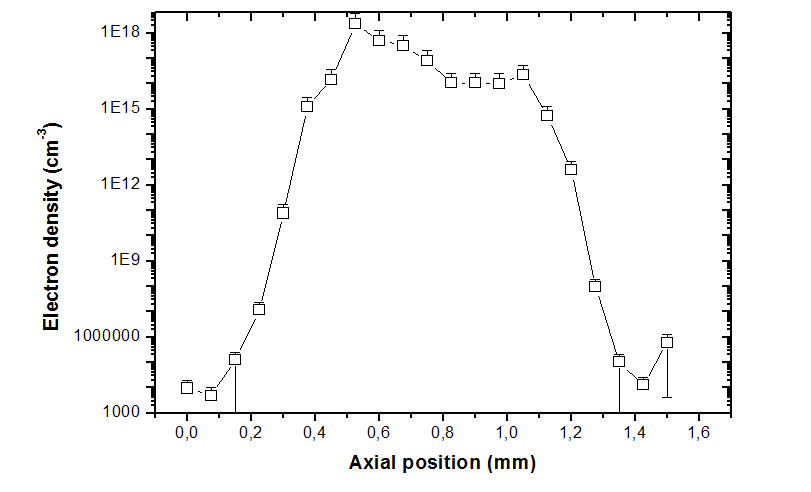

Figure 4 Electron density distribution. 4a Total electron density of plume along expansion axis,6 and 4b time evolution of electron population of 4d45s6s state.5
Usually plasma electron density is determined by Stark broadening
, which expression depends of electron impact width parameter
, according to Equation 3.
(3)
However, database for molybdenum in literature is scarce, and this electron parameter is unknown. If it can be reputable that the experimental line broadening is mainly due Stark and instrumental broadening mechanisms fitted by lorentzian profile and, once electron density was determined by Saha-Boltzmann equation, Stark broadening expression permits to determine the electron impact width parameter. For 273.339 nm molybdenum plasma emission line expanding on air at atmospheric pressure and temperature nearly 8000 K, the electron impact width parameter was measured equal to (0.017±0.008) nm.6
Plasma expanding velocity can be determined as the ratio between length of laser scattered light equal to (0.35±0.05) mm and laser pulse duration equal to (75±5) ns, considering that the plasma expansion is uniform. So, the calculated plasma expansion velocity is (5.0±0.7) km/s and, with measured plume length equal to (1.80 ± 0.05) mm, plasma lifetime is determined equal to (160±14) ns. Table 1 summarizes molybdenum plasma properties of laser-ablated plume expanding on air at atmospheric pressure and it presents a comparison with reference data.8–12
|
Atmospheric pressure |
Mo references |
References for other metals |
Expansion velocity |
5.0 km/s |
--- |
10.0 km/s |
Plume length |
1.8 mm |
--- |
2.0 mm |
Electron temperature |
15000 K |
10000 K |
10500 K-13000 K |
Electron density |
2.2 × 1018 cm-3 |
5.0 × 1016 cm-3 |
1016 cm-3-1018 cm-3 |
Electron impact width parameter |
0.017 nm |
--- |
0.015 nm |
Table 1 Molybdenum plasma properties
Conclusion
Emission spectroscopy study of laser-ablated Mo plasma along plume expansion axis was performed. Plasma parameters as plasma expansion speed value around 5.0 km/s and plasma lifetime nearly 150 ns was calculated using uniform and one-dimensional expanding model. Due direct comparison between experimental and theoretical spectra and emission lines assignment, it was demonstrated that the plume evolves from the atoms mainly at ionized states, near target surface, to the atoms at ground state at the end of plume emission, so that the plasma becomes a neutral, monatomic and at ground state Mo jet.
Considering Local Thermodynamic Equilibrium Model and Saha-Boltzmann equation, electron temperature was calculated around 15000 K, reaches its maximum value at central portion of the plume and decays at the boundaries. Also, electron density reaches values of 1018 cm-3 and electron impact width parameter was determined by Stark broadening expression equal to (0.017±0.008) nm for temperature of 8000 K.
The obtained results reveal laser ablation as an auxiliary method for isotope separation methods and that laser-ablated Mo plasma plume could be used for 99Mo production.
Acknowledgments
Conflicts of interest
Authors declare that there are no conflicts of interests.
References
- Soonawala D, Amin T, Ebmeier KP, et al. Statistical Parametric Mapping of 99mTc-HMPAO-SPECT Images for the Diagnosis of Alzheimer’s Disease: Normalizing to Cerebellar Tracer Uptake. NeuroImage. 2002;17(3):1193–2002.
- Verbeek P. Report on molybdenum-99 production for nuclear medicine – 2010-2020. Association of Imaging Producers & Equipment Suppliers. 2008.
- Matos JB, Oliveira ML, Sternberg EMA, et al. Generation of an Atomic Beam by Using Laser Ablation for Isotope Separation Purposes. Journal of Aerospace Technology and Management. 2012;4(4):413–420.
- Sternberg EMA, Rodrigues NAS, Sbampato ME, et al. Excited states time evolution on a laser-ablated molybdenum plume. Applied Physics B. 2014;116(4):985–989.
- Sternberg EMA, Rodrigues NAS, Amorim J, et al. Plasma properties of 355 nm and 532 nm laser-ablated molybdenum target at atmospheric pressure. Journal of Physics: Conference Series. 2012;370(1):1–8.
- Sternberg EMA, Rodrigues NAS, Amorim J. Molybdenum electron impact width parameter measurement by laser induced breakdown spectroscopy. Applied Physics B. 2016;122(21).
- http://www.nist.gov/physlab/data/asd.cfm accessed at 21/11/2012
- Jakubowska K, Kubkowska M, Blagoev A, et al. A spectroscopic study of laser ablation plasma from Mo target. Physica Scripta. 2014.
- Hanif M, Salik M. Optical emission studies of molybdenum plasma produced by an Nd: YAG laser. Journal of Russian Laser Research. 2014;35(3):230–238.
- Farid N, Wang H, Li C, et al. Effect of background gases at reduced pressures on the laser treated surface morphology, spectral emission and characteristics parameters of laser produced Mo plasmas. Journal of Nuclear Materials. 2014;438(1-3):183–189.
- Farid N, Harilal SS, Ding H, et al. Emission features and expansion dynamics of nanosecond laser ablation plumes at different ambient pressures. Journal of Applied Physics. 2014;115(3).
- Lide DR. CRC Handbook of Chemistry and Physics 87th edn. CRC Press, Boca Raton, USA. 2006.

©2017 Sternberg, et al. This is an open access article distributed under the terms of the,
which
permits unrestricted use, distribution, and build upon your work non-commercially.





