MOJ
eISSN: 2475-5494


Case Report Volume 10 Issue 4
Obstetrics and Gynecology Residency program, Al Ain hospital, United Arab Emirates
Correspondence: Dr. Nourah Alkindi, Senior medical OBGYN resident, Al Ain hospital, Al Ain, United Arab Emirates, Tel 00971561052232
Received: June 20, 2021 | Published: September 2, 2021
Citation: Al Kindi N, Kapadia S, Alam AR. Successful obstetric outcome in a case of severe idiopathic aplastic anemia. MOJ Womens Health. 2021;10(4):102-103. DOI: 10.15406/mojwh.2021.10.00297
Pancytopenia is reduction of all three cell lines (myeloid, erythroid and megakaryocytic). The clinical manifestations are secondary to reduced cell lines leading to pallor, fatigue, dyspnea, bleeding or bruising or fever. Pancytopenia can be secondary to nutritional deficiencies (B12), auto immune disorders, bone marrow failure syndromes, replacement of marrow (secondary neoplasia) or malignant hematopoietic diseases (Acute leukaemia).1 Pancytopenia in pregnancy is rare and can possibly lead to both maternal and fetal morbidity and mortality.2 In this report we present a young female who presented with pancytopenia secondary to aplastic anaemia and had a successful maternal and fetal outcome.
A 27years old Egyptian female, G2 P1+0 (previous one normal delivery) was referred to our hospital at 30+5weeks with suspected fetal anomaly. She had an uneventful antenatal care and had no significant past medical or surgical history apart from family history of thrombocytopenia. Ultrasound scan confirmed a small left renal cyst, with normal growth and liquor. She denied any history of drug exposure or allergies. Routine blood tests suggested a low platelet count of 65X10^9/L with a haemoglobin (Hb) of 97g/dL, therefore she was admitted for further investigations. Subsequent blood investigations showed further decline in the platelet count to 6x10^9/L and borderline vitamin B12 level. Blood film was suggestive of severe thrombocytopenia (likely to be of immune type). Her blood group was AB positive.
She was treated with Intravenous immunoglobulin, methylprednisolone and six units of platelets transfusion (1 unit of donor apheresis) as recommended by the haematologist. which she initially responded well, but after 3days, she started complaining of weakness and dizziness and her repeat investigations showed WBC 1.9 X10^9 /L, Hb 85g/dL, Platelets count of 13 X10^9/L. The patient was referred to a tertiary care hospital with haematology oncology service. On initial evaluation she was pancytopenic. Furthermore, there was borderline vitamin B12, no coagulopathy and negative autoimmune work up. Patient had bone marrow aspirate with cytogenetic testing. Bone marrow aspirate showed severe hypoplasia with cellularity less than 5%. (Figures 1&2) There were no chromosomal abnormalities detected. Paroxysmal Noctural Hemoglobinuria (PNH) clone was less than 1 %. Patient was not tested for Fanconi's anemia or telomeropathies.
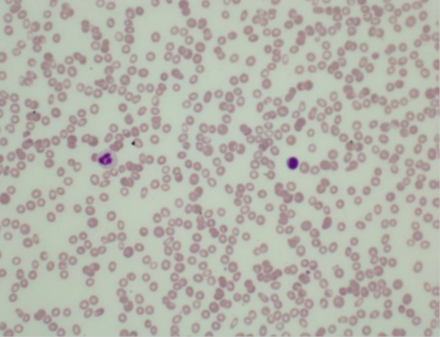
Figure 1 Blood film shows severe thrombocytopenia and leukopenia (Probably immune type) and hypochromic anaemia.
As she was pregnant supportive care was maintained with packed red cell transfusion along with platelets.
The transfusion parameters are haemoglobin less than 8 g/dL and platelets less than 20 X10^9/L.
Patient was induced at 34weeks in view of progressive worsening of neutropenia WBC 0.6 x10^9/L, thrombocytopenia 12 x10^9/L and Hb of 8.2g/L, despite aggressive supportive management. Before induction, two units of erythrocytes, three units of platelets were transfused along with intravenous steroids, which improved her platelets count to 55 x10^9/L, Hb 11 g/dL and WBC 2 x10^9/L. She received prophylactic antibiotics during labour and had an uncomplicated normal vaginal delivery of a healthy female child weighing 2.8Kg with good Apgar score.
After delivery, the blood investigations showed WBC 2 X10^9 /L, Hb 80g/dL and platelets count 60X10^9/L. She received granulocyte colony -stimulating factor (G-CSF) with improvement in the blood picture and was discharged home on day 10 with follow up in hematology clinic.
Six weeks postpartum, she was seen in the haematology oncology clinic and her labs showed increased WBC to 2.6 x10^9/L with an Absolute Neutrophil count (ANC) of 0.98 with filgrastim support, hemoglobin was 10g/dL and platelets count had improved to 65 X10^9/L spontaneously. Six months postpartum, the patient was asymptomatic and reported no episodes of bleeding or infection, her investigations showed normal level of WBC, haemoglobin and platelets count (180X10^9/L).
Aplastic anaemia (AA) is a rare acquired or inherited haematological disorder resulting from the failure of haemopoiesis with an annual incidence of 2 to 6/1.000.000.3 Patients may present with fatigue, bleeding due to thrombocytopenia and recurrent infections due to neutropenia. The most common cause of aplastic anaemia is idiopathic, but it can be triggered by T-cell mediated auto-immune disease, drugs, viral infection, toxins and pregnancy.3-5 The correlation between AA and pregnancy is still unclear; despite that, a small contribution of pregnancy to the development of severe aplastic anemia cannot be excluded.3,6
The initial presentation of pancytopenia should include investigations to rule out reversible causes such as vitamin B12 or folate deficiency or medically manageable diseases such as connective tissue disorder. The subsequent work up should follow the international guidelines,7-10 which should be thorough including a bone marrow biopsy with cytogenetic evaluation.
Furthermore, the treatment of aplastic anaemia in pregnancy should be by a multidisciplinary-team approach to coordinate prenatal care, optimize maternofetal outcomes, and plan peripartum interventions.1 In non-pregnant women, the choice of therapy depends on the age, performance status, presence of comorbidities and availability of HLA identical stem cell donor.8-10
It can be broadly categorized into either hematopoetic stem cell transplantation or immunosuppressive.11 Comparing to a pregnant patient, the choice of therapy is limited to supportive therapy by transfusion support or immunosuppressive therapy (IST) depending on the stage of pregnancy. Supportive therapy is consider as the first choice of treatment in pregnant women with AA, with recommended hemoglobin levels of more than 8g/dl and platelet count of more than 20*109/L. Additionally, there are reports of patients successfully treated with corticosteroids, cyclosporine, and Granulocyte colony-stimulating factor
In Aplastic anemia, the benefit of corticosteroid is limited compared to immuno-related causes of cytopenia that result from cell destruction (eg, hemolytic anemia and idiopathic thrombocytopenic purpura).2 The used corticosteroids have no ability to cross the placenta, such as prednisone, prednisolone, and hydrocortisone, to decrease the risks of fetal brain exposure and orofacial malformations. BJH guideline, 2015, for the diagnosis and management of adult aplastic anaemia recommended the use of cyclosporine in pregnancy if needed, but some studies reported, it may lead to neutrophil count elevation, severe thrombocytopenia, premature delivery and low-birth -weight infants in pregnancy. In view of that, cyclosporine has not been shown to be consistently effective and it should be carefully used.2,9 Furthermore, GCSF was shown to be a safe and effective therapy in a retrospective analysis of 38 pregnant patients, and can be recommended when the disorder is accompanied by significant neutropenia. Our patient responded well to GCSF treatment but not responding to an aggressive supportive therapy.2
Treatment of severe aplastic anemia in pregnancy is challenging, as it needs to encounter maternal and fetal benefits and risks carefully before starting treatment. Current therapeutic options of Immuno-suppressive therapy with Anti-thymocyte Globulin (ATG) and cyclosporine are not without maternal and fetal risks and require a prolonged time period before efficacy is observed with transfusion independence.2,9 Similarly, thrombopoetic agent, Eltrombopag is not approved for use in pregnant patients.9 This case represents a good maternal and fetal outcome in severe aplastic anemia with a multidisciplinary-team support, aggressive supportive therapy and GCSF treatment. There was spontaneous recovery post-delivery.9
None.
None.

©2021 Al, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.