MOJ
eISSN: 2475-5494


Research Article Volume 9 Issue 2
Department of Histology, University of Campinas, Brazil
Correspondence: Eliana Mara Oliveira Lippe, Department of Histology, Ninth of July University (UNINOVE), Bauru, SP, CEP 17011- 102, Brazil
Received: April 10, 2020 | Published: April 23, 2020
Citation: Oliveira LLD, Lippe EMO. Nitric Oxide levels and the uNK cell aspects as clinical predictors in induction–inflammation model during pregnancy and neurological problem. MOJ Women’s Health. 2020;9(2):50-57. DOI: 10.15406/mojwh.2020.09.00269
The successful of pregnancy in humans and rodents occur between the interaction maternal and fetal interface, specially involving the participation of uNK cells. This interaction involved neo angiogenesis, placentation and presence of mediators like nitric oxide. During the pregnancy the administration of LPS in the dams can results in necrosis, preterm birth, IUGR, miscarriage or neurological problem. Once the uNK cells are activated, they can produce vasodilators, like NO. So, the main purpose of this study was to evaluate if LPS cause alteration in the uNK cells in pregnant mice and if the same behaviour can be detected by NO in the blood. Also we evaluated the effect of LPS to cause neurological injuries. To do that we used pregnant mice on gd 10th and those was treated with LPS for different times. Uterine samples were collected at 0.5,1,2 and 6hr after LPS treated and processed for paraffin embedding and tissue homogenate. The samples designated for paraffin embedding was performed the Dolichos biflorus (DBA) lectin cytochemistry and anti-iNOS immunocytochemistry. The samples designated to tissue homogenates were processed for SDS-PAGE and Western-blot using anti-iNOS and evaluate of NO concentration. We found after 2h LPS exposure the mice showed fever and low capacity to explore different environment. At the same time, we found increase in the nitrate/nitrito ratio in a dose dependent manner in the uterus after 2h LPS exposure. The uNK cells were the main cell that was staining for iNOS isoform. Also, we found that wall:lumen ratio is very higher in treated mice than the control mice. The LPS is able to induce the activation of uNK cells and this action is involved by releasing NO in higher amount. So, it is possible to consider the uNK cells as a potential element of maternal-fetal interface in the production of NO and knowing that the isoforms is reduced in these cells, a model of NOS inhibition could be considered to elucidate the participation of uNK cells as a possible cause of effectors loss or interruption of pregnancy.
Keywords: LPS, uNK cells, Pregnancy, NO concentration
Epidemiological data have established that fetal or intra-amniotic infections are associated with introduction of fetal proinflammatory cytokines and development of neurologic injury in preterm and near-term infants.1 Despite these findings, the majority of cerebral palsy is still unexplained by either fetal infection or hypoxia. One of the mechanisms involved to start infection is by LPS (lipopolysaccharide). The LPS can bind to Toll-like-4 receptor in macrophages and start the cascade through NFκβ).2 During the pregnancy, the administration of LPS in the dams results in necrosis, preterm birth, IUGR or abortion.3,4 In the pregnancy, the successful in humans and rodents occur into the interaction between maternal and fetal interface. These ideas involve deep alteration in the uterine environment, like neo angiogenesis, placentation and presence of mediators like nitric oxide.5 In the uterine environment of pregnant mice was observed the migration of uNK cells.6,7 In humans, we can find uNK cells around second half of ovarian period.8 In mice, the uNK cells appear around 5 gestational days (gd) and increase in number after placentation period, around gd12. In the period closer to partum the number decrease.9,10 The uNK cells are able to produce a variety of cytokines, like IL-1; LIF; CSF-1; TNF-α, IFN-γ. Wu et al.,10; Cascado et al.,3 and NO Ogando et al.,11 The nitric oxide (NO) is a free radical originated from enzyme Nitric Oxide Synthase (NOS).12 It is involved in many physiological and pathophysiological mechanisms. In reproduction the NO act in the foliculogenesis, tissue remodulation.12 When NO is present at beneficial concentration the effect to the cells contributing to perform the most of the functions, however, when appearing in excess, the deleterious effect contribute to cause damage in the tissue in general. There are three different isoforms that can form NO: iNOS, eNOS and nNOS. iNOS isoform is inducible type and require constant stimulation to be produced. The main function in the pregnant uterus are maintain low vascular resistance and act as vasoconstrictor, moreover, can prevent platelet adhesion and neutrophil aggregation and the surface of trophoblast cell.13 Ogando et al.,13 demonstrated that pregnant mice increased expression of iNOS during estrus and proestrus and this could be due to interaction with steroids hormones like estrogen, progesterone. Since NO could be deregulated in the end of pregnancy, one feature that many occur due to dysfunction of adequate vascularization, like preeclampsia, which is a multisystem disorder of pregnancy that affects approximately 5-20% of the population Gajewska et al.,14). This situation results from endothelial dysfunction and inappropriate trophoblastic invasion of the uterine spiral arteries, reducing placental perfusion with the involvement of NO in the regulation of these arteries.
The purpose of this work was to evaluate if LPS can cause alteration in the uNK cells in pregnant mice and if the same behaviour can be detected by NO in the blood. Also we evaluated the effect of LPS to cause neurological injuries.
Animals
Swiss mice were purchased from CEMIB/UNICAMP. The animals were bred and maintained with temperature and light under control with free access of food and water. All protocols were approved by CEMIB (protocol. no. 1144-2). Time of pregnancy was verified by visual inspection of vaginal plug which was deemed gestational day (gd) 1. All mating experiments were repeated at least three times which at least three mice/treatment.
Animals treated with Lipopolissacharideo (LPS)
The animals on gd10th were inoculated intraperitoneally with 0.5-1µg/animal of LPS (LPS-Escherichia coli 05:B55 and 026:B6 - Sigma Chemical Co, USA) diluted in 100μl de 100mM PBS pH 7.4. The control group was treated with the same amount of 0.3% saline. The animals were sacrificed after 0.5, 2 and 6h of LPS treatment.
Behavior study
We evaluate the effects of LPS in the establishment of behavior. All pregnant animals on 10th gd was submitted the open field behavioral test, light dark box test (Abe et al.,1) and forced swimming test.9 In addition, we checked the temperature of all animals. Behavioral assessments of anxiety parameters, depression and memory were performed in the periods of 0.5, 1, 2 and 6 hours after treatment with LPS. Others mice received only intraperitoneal saline injection as control and these had their behaviors analyzed by the same apparatus used in the experimental group. All of these animals were subjected to the same tests, that is, respectively the test of the cross maze, followed by the object recognition test and finally the forced swim test. To detect the antidepressant effect, the forced swim test was used (“Forced swimming test”). This test was developed by Porsolt; Bertin and Jalfre9 for research with antidepressant drugs. For this, a cylinder was used vertical glass, with dimensions of 14 cm in diameter and 25 cm in height, filled with water at 30ºC up to a height of 20 cm. The volume of water should allow that the animal can swim or float (“float”) without touching its feet or tail on the bottom of the container. For the test, each mouse was placed in the cylinder for 5 minutes and the latency time with which the animal exhibits floating behavior and the total floating time (time when the animal makes small movements just to keep your head above water level). Only the last 4 minutes have been analyzed, according to the Porsolt methodology.
Cytochemistry and Immunocytochemistry
At least, 3 animals from each experimental group and corresponding control animals were perfusion fixed with 30ml of 4% paraformaldehyde (PFA) in 100mM PBS pH 7.4 through the left ventricle. The uterine fragments containing embryo development sites were processed according to the conventional paraffin embedding and 5μm thick paraffin sections were collected on silanized glass slides. Dolichos biflorus (DBA) lectin cytochemistry was performed according to Paffaro Jr. et al., (2003). Briefly, desparaffinized sections were blocked with 1% bovine serum albumin (BSA type V, Sigma Chem. Co., St Louis, USA) in 100mM PBS pH 7.4 and incubated with biotinylated DBA lectin (Sigma Chem. Co., St Louis, USA). After treatment with streptavidin-peroxidase (Chemicon, USA), the peroxidase was revealed with 0.5% diaminobenzidine (DAB) and hydrogen peroxide (H2O2). There were employed, on paraffin sections, immunoperoxidase using the mouse monoclonal anti-iNOS diluted in the 0.5µg/µl concentration (1:200) (Sigma Chem. Co., Louis, USA) as primary antibody and peroxidase conjugated rabbit anti-mouse (Santa Cruz, USA) as secondary antibody. After revelation with DAB and H2O2, the sections were counterstained with hematoxylin and mounted with synthetic Canada balsam under coverslips. In addition, wall arteries and diameter of lumen of each blood vessel were quantified. We used 15 sections of each experimental group and control. The thickness of the wall was calculated as follow: (diameter of the wall)/2. The calculation was got using Zeiss AxioVision Sofware (versão 4.8). The statistical analysis was made using Prism 4.0.3 Statistical Software (Graph Pad) with significance level. Data were analyzing using ANOVA with Bonferroni test where p<0.05 was considered significant.
Tissue Homogenate The mesometrial lymphoid aggregate of pregnancy (MLAp) region was dissected from 2 embryo lesioned sites of 3 different animals of each experimental group. These tissue samples in ice cooled bath were homogenized in 10mM Trizma base (Sigma Chem. Co., St Louis, USA) pH 7.4 containing 10mM EDTA (Sigma Chem. Co., St Louis, USA), 10mM sodium pyrophosphate (Sigma Chem. Co., St Louis, USA), 100mM sodium fluoride (Sigma Chem. Co., St Louis, USA), 10mM sodium orthovanadate (Sigma Chem. Co., St Louis, USA), 2mM PMSF (Sigma Chem. Co., St Louis, USA), 0.1mg/ml aprotinin (Sigma Chem. Co., St Louis, USA) and 1% igepal (Sigma Chem. Co., St Louis, USA) using Glass- Col (USA) homogenizer. The homogenates were centrifuged (12000g) for 30 min at 4º C and the protein contents in the supernatants were evaluated according to Bradford et al.,16
Nitrate and Nitrite assay (NO)
The sample of MLAp region were colleted was performed according to the item 2.5 and was additionally centrifuged with centricon (Amicon, Inc, USA) during 30min, 12000g at 4oC and storage to -70oC until the moment of use. The sample was diluted 1:10 in 100mM PBS pH 7.4 to determine the amount of nitrate and nitrite by colorimetric test (Cayman Chemical, USA) and the reading was made in Reader of Elisa (Molecular Devices, California 94089) and the analyses was made in program SoftmaxPro between 540 and 550nm. The sample was compared with standard curve of nitrate and nitrite. The statistics analyses of averages ±SD and the degree of significance were made by ANOVA test. The blood collection was performed after animal was sacrificed by cervical dislocation. The blood was collect by exsanguination from the heart and following by centrifugation with 1600g for 15min at 4oC to collect the plasma and another centrifugation for 20000g for 30min at 4oC to collect the supernatant and quantification the nitrate and nitrite as we describe above.
Behavior tests (Figure 1)
183 Septic shock is a challenge for medicine, since it is responsible for a very high mortality rate, even with adequate treatment based on antibiotics and vasopressors. This unhealthy state is characterized by vasodilation peripheral arteries, which results in decreased systemic vascular resistance, high cardiac output, hypotension and inadequate tissue perfusion in response to bacterial products,17 such a state therefore, it can be quite harmful in pregnant women who need of adequate uterine perfusion and the exposed pregnant mouse model exposed the LPS can assist in clarifying the mechanisms involved in this process. The literature points out that the shock caused by bacterial endotoxins is accompanied by changes in body temperature, based on this; in the present study all animals used had their temperature measured. The pregnant mice on the 10th gd without any treatment showed an average of 37.18oC of body temperature. This average showed no difference significant (p> 0.05) when compared to the average obtained from the experimental group where saline was administered (37.05oC). Shortly after the administration of E.coli LPS, the body temperature of the pregnant mice decreased (p <0.01) in such a way that after 1 hour it reached average of 35.67oC. After this initial drop, the temperature not only rose again as it exceeded the temperature observed in normal pregnant mice, reaching averages of 37.65oC after 2 hours of application of LPS and 38.24oC after 6 hours (p <0.05). The treatment with LPS induces different symptoms, like fever, anxiety and depression. After 2h of exposed to LPS, the mice showed fever (graph 1), anxiety behavior (graph 2) and depressive behavior (graph 4) in pregnant mice. In addition, the mice showed loss of capacity to explore different environment (graph 3).
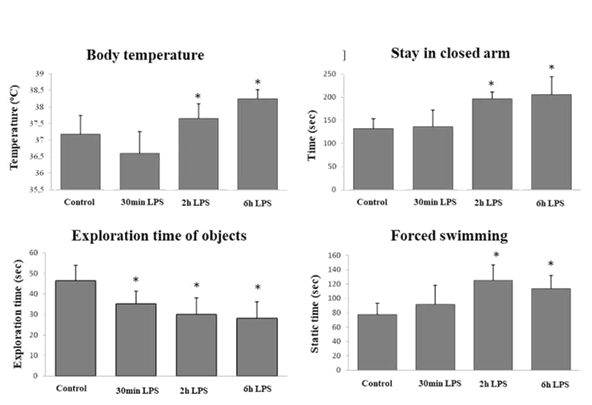
Figure 1 The treatment with LPS induces different symptoms, like fever, anxiety and depression. After 2h of exposed to LPS, the mice showed fever (graph 1), anxiety behavior (graph 2) and depressive behavior (graph 4) in pregnant mice. In addition, the mice showed loss of capacity to explore different environment (graph 3).
A reduction in the exploratory capacity of the arms of the labyrinth was observed comparing the behavior of animals treated with LPS with the presented by pregnant animals on the 10th gd without any treatment (p <0.05). In addition, the rates of stay in the arms indicate that after 30 minutes of LPS administration, the animals did not show altered behavior in parameters related to anxiety (p> 0.05), and also there is no significant difference when comparing the normal group (10th gd) with the group control. However, from 1 hour after application of LPS, animals demonstrated increasing anxiety behavior (up to 6 hours after application), diagnosed by the increase in the rate of permanence of these animals in the arms closed concomitant to the decrease in the rate of stay in the arms open, demonstrating LPS anxiogenic action in these animals. The results obtained through the Forced Swimming test made it possible to identification of the onset of depressive behavior already expected by the induction of inflammation by LPS. Two hours after the application of the LPS there is an abrupt increase in the time that the animals remain.
Morphological analysis of implantation sites
All animals showed unhealthy behavior after 0.5h, so the next step was evaluated the implantation sites and we observed the hyperemic regions, but not hemorrhage with some necrosis in the latest period.
Quantification of nitrate and nitrito in the uterus (NO) (Figure 2)
The graphs showed the ratio between nitrate (blue bar) and nitrite (purple bar) quantification in the figure 2. After 30 min of LPS treatment we observed decreased of nitrate/nitrito ratio and increased nitrite after 2h of LPS treatment and start decrease again after 6h.

Figure 2 The graph showed the ratio between nitrate (blue bar) and nitrite (purple bar) quantification. The nitrite quantification increased after 2h of LPS treatment and decreased after 6h.
Immunocytochemsitry for iNOS and Cytochemistry of DBA lectin (Figure 3)
The iNOS isoform is an inducible isoform, but different that was found in literature, during pregnancy, the iNOS is express constitutively. The main source was present in the myometrium fibers, in the cytoplasm of trophoblast giant cells and diffuses staining in the cytoplasm of uNK cells nearest of the cellular surface. Some of the subtypes of uNK cells were not reactive to iNOS. After 2h of LPS treatment, we observed more staining in the cytoplasm of immature uNK cells and decrease in mature uNK cells. Considering the period of 6h, the staining was weaker in the immature uNK cells and was observed more staining in mature uNK cells. The reactivity of DBA lectin in LPS treated animals was different that was observed with control animals. The staining was not continuing in the cellular surface and with partial absence or total absence of the granules after 2h of LPS exposure. The morphological analysis of animals treated with LPS for 2h showed dilatation of blood vessels and some signals of tecidual degradation with significative leukocyte invasion. This partner was described for 0.5 and 2h after treatment with LPS. In 6h we observed increase in the reactivity of uNK cells with DBA lectin.
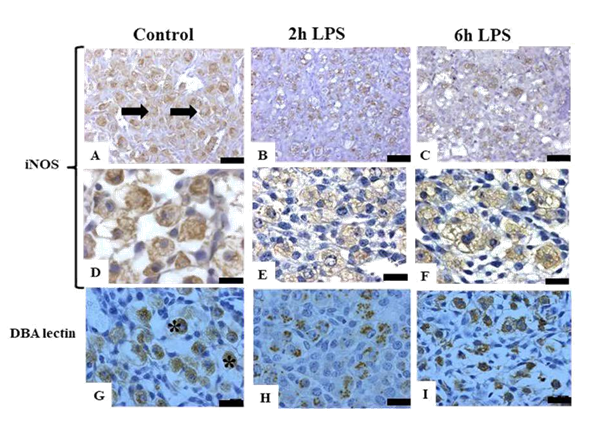
Figure 3 Photomicrographs of anti-iNOS immunoperoxidase reaction in pregnant mouse uterus on 10th dg, counterstained with hematoxylin in control animals and LPS treated animals (2h and 6h) A and D) anti-iNOS positive reaction in the cytoplasm of uNK cells (arrows) in the uterus of normal pregnancy; B and E) anti-iNOS positive staining decreased after 2h of LPS treatment; C and F) anti-iNOS positive reactions start to increase after 6h of LPS treatment; G) DBA lectin showed uNK cells staining in the granules and surface (*); H) uNK cells decreased staining of DBA lectin in the granules after 2h of LPS treatment; I) uNK cells start to increase DBA lectin staining after 6h of LPS treatment. Bars=30μm and 50 μm.
Morphometric analysis of vessels (Figure 4)
The next step was evaluating the effect of NO in the alteration of vascularization and vascular permeability. For this purpose, we checked the diameter of vessels according to Croy et al.18 The wall:lumen ratio was higher in treated animals than our control animals.
The mechanisms involving the dialogue between maternal and fetal side is not completely understood. The development of different experimental model that mimics abnormal pregnancy is helping to understand the mechanism that control homeostasis uterine. Our data showed that uNK cells are mediators in this inflammatory response after LPS treatment and most of the case involves loss of pups. However, it is not sure that uNK cells can respond directly to that stimulus. According to Casado et al.,3 the effects that LPS cause in the pregnant uterus are necrosis, fetal death or abortion and cause some alteration into the nervous system. Our data showed that, similar that we found in non-pregnant mice, there is unhealthy behavior after LPS treatment in pregnant mice. According to Salter et al.,19 the stimulus from endotoxin result in increase of expression genes of NOS that produce nitric oxide in the peripherical tissue, like brain. Ribero et al.,20 showed that administration of L-NAME (L-nitro-methil-ester) before LPS treatment was able to reduce the effects of LPS related to unhealthy behavior. In addition, the L-NAME treatment can increase the levels of corticosterone and this suggests that NO can be developed in the unhealthy behavior. The principal morphological alteration observed in these animals was related to the vascularization, including hyperemic in the short period. These data suggest that some vasodilator is involving in this process. Between the vasodilators that were present in the uterus, we observed that nitric oxide (NO) was present in different periods of pregnancy. They can act during relaxation of uterus and partum. However, the NO also show incredible toxic potential when in higher doses and it is capable to influence different processes, like lipidic peroxidation, DNA break, inhibition of mitochondrial pathway.21 The production of NO is time-dependent, which means elevated during pregnancy and decrease closer to the partum.22 Thus, the level of NO should be approximately constant for the success of embryo development and decrease in the concentration of NO can be indication of end of pregnancy. The amount of NO present in the pregnant uterus is produced by enzymes NOS. There are three isoforms of NOS and all of them are constitutive present in the uterine environment.13 Among the various cell types present in the uterine environment, uNK cells show potential to produce NO. Therefore, target cells were uNK cells in this study and we try to elucidate the possible source of the vasodilators agent involved at the changes previously described. By DBA lectin cytochemistry, the changes were observed in the pattern of surface and granules of uNK cells and indicate that probably the NO is to be released. The main alteration has been determined in the shorter periods of treatment. The results indicate rapid mobilization of uNK cells to bad stimulus, with possible release of the granule content and production of free radicals, which can cause degeneration in a longer period of treatment. The reports about the possible involvement of NO observed in the uNK cells were the basis of quantification of this radical. For this purpose, we used homogenized maternal tissue, composedly mainly of uNK cells in order to quantification the NO. The results indicated the group treated with LPS showed peaks after 0.5h of NO. To better understand the events observed in the pregnant mice, we detected the presence of iNOS to identify a possible source of NO. The results showed absence of staining for iNOS in all treatment, especially after 0.5h, suggesting that isoform is quickly consumed as the same NO. But the NO can be produced and consumed by others isoforms. During the periods that groups were treated with LPS showed the presence of NO with peak of concentration not in accordance with the period of maximum isoform expression. Thus, it can be assumed that NO is produced on a larger scale by the constitutive isoforms, like iNOS, since the peak of NO are still lower than the amount present in the control group. To determine the actual action of the changing levels of NO was adopted a model for inhibition of free radical-producing enzymes. One of these inhibitors is the L- NAME, an analogue of L-arginine can act in three isoforms of NOS. This inhibitor competes with the natural substrate of the enzyme blocking its active site. His performance 309 in the model of LPS treatment justified its use in an attempt to reduce fetal loss (Atanassakis et al.,23 Benencia et al.,24). Studies by Croy and collaborators (2005) demonstrated that the low dose of LPS can alter iNOS expression, however, not enough to cause immediate morphological changes in a pregnant uterus of mice. However, already that LPS is able to alter the production of pro- inflammatory cytokines, as well as the expression of adhesion molecules (such as selectins) that favor migration and diapedesis of immune cells, it is expected that changes in the number and in the activity of uterine Natural Killer cells. Therefore, according to our results it is possible that the pregnancy in mice is able to prevent short-term memory loss after LPS-induced sepsis. However, it should be noted that the methodology used by Barichello and collaborators25 was not similar to that developed in this work, the sepsis was induced by PLC and only 10 days after surgery the animals underwent behavioral testing. Another behavior identified as a component of unhealthy behavior that could be altered when the treatment is depressive. As described previously, the animals of each experimental group were submitted to the Forced Swimming to assess the occurrence of possible depressive behavior. The result suggests that these animals are depressive, and this change confirms the establishment of unhealthy behavior. The depressive state was evident from the second hour after treatment and persisted until the last evaluated period. The results suggest a latency period 2 hours for the depressive behavior to establish itself in the individual, the same latency time (2 hours) was also observed for the feverish state. Changes in the immune system appear to precede the development of depressive state, in addition, this state is related to the increase in serum levels of cytokines, demonstrating the importance of these molecules in mediating emotional and cognitive responses to infection. Our data obtained by the present study demonstrate that the mouse organism pregnant against this dose of LPS, with respect to the development of fever, has a latency of approximately two hours. These results corroborate those found by Giusti-Paiva and collaborators17 who studied male rats, and attributed that the alteration of body temperature is also due to the biphasic behavior of the levels of vasopressin during septic shock. In this study it was observed that even the two hours after the administration of LPS there was an increase in the levels of vasopressin concomitant with drop in temperature. After two hours it was verified temperature increase in these animals and the levels of vasopressin decreased. So we can consider the uNK cells as a potential element of maternal-fetal interface in the production of NO and knowing that the isoforms is reduced in these cells, a model of NOS inhibition could be considered to elucidate the participation of uNK cells as a possible cause of effectors loss or interruption of pregnancy.
We would like to thank Dr. Aureo Yamada from the University of Campinas (UNICAMP, Brazil) for the help with the manuscript preparation and Dr. B. Anne Croy (Queen`s University, Kingston, Canada) for valuable and critical revision of this manuscript.
This study was supported by Federal Agency for Support and Evaluation of Graduate Education-CAPES, Brazil, grant n. 4683-08-0.
The author declares that have no conflict of interests.

©2020 Oliveira, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.