MOJ
eISSN: 2475-5494


Research Article Volume 3 Issue 2
1Department of Obstetrics and Gynecology, Al Hussein Hospital, Al Azhar University, Egypt
2Department of Obstetrics and Gynecology, Al Amreya General Hospital, Alexandria, Egypt
Correspondence: M Samir Khalaf, Department of Obstetrics and Gynecology, Al Hussein Hospital, Al Azhar University, Cairo, Egypt
Received: August 09, 2016 | Published: December 20, 2016
Citation: Khalaf MS, Mira I, Farahat M, et al. Comparison between haptoglobin and CA125 to predict nature of ovarian mass. MOJ Womens Health. 2016;3(2):201-205. DOI: 10.15406/mojwh.2016.03.00065
Background: Ovarian cancer has been named "the silent killer" because it frequently causes non-specific symptoms, which contribute to diagnostic delay, diagnosis in a late stage and poor prognosis. Despite of the improved surgical techniques and effective chemotherapeutic regimens available for management of ovarian cancer, there is no improvement in its early detection. Thus ovarian cancer remains a leading cause of death from gynecological malignancies with an overall poor prognosis. The identification of novel cancer biomarker opens the possibility for early detection, better monitoring of tumor progression. Haptoglobin is a novel biomarker which was used in diagnosis in ovarian cancer.
Objectives: To assess the performance of haptoglobin and CA125 to increase the sensitivity and specificity in diagnosis of nature of ovarian mass and to assess the diagnostic value of haptoglobin in cases of ovarian neoplasia.
Methods: This study was carried out on 90 women attending El-Hussein and Bab El-Sheria University Hospital, a prospective study was held on patients with ovarian mass prepared for surgery. It is a case control study. It incorporated on 60 patients with ovarian masses and 30 women without masses. Those patients were subjected to careful history taking, clinical examination, ultrasound examination and preoperative assessment of serum levels of Haptoglobin and CA125.
Results: Analysis of CA-125 as a single biomarker using calculated cut off value>36U/ml shows that the sensitivity was 90.0%, specificity 92.0% in the diagnosis of malignant ovarian tumors with p value=(0.002). Analysis of Serum haptoglobin as a single biomarker using calculated cut off value>1.9mg /ml shows that the sensitivity was 88.0%, specificity 85.0% in the diagnosis of malignant ovarian tumors with p value=(0.0016). Analysis of both Serum CA-125 and haptoglobin biomarkers using calculated cut off value>36U/ml and>1.9 mg/ml respectively shows that the sensitivity of both was 94.0%, specificity 93.0% in the diagnosis of malignant ovarian tumors with p value=(0.001).
Conclusion: Haptoglobin has an important role in the evaluation of ovarian cancer and its serum level increases in early stages of cancer ovary. It increases significantly in malignant ovarian tumors than in benign ovarian tumors, so it can be used as a diagnostic tumor marker in cases of ovarian neoplasia so haptoglobin is a novel tumor marker for ovarian cancer with accuracy near to that of CA125.
Keywords: ovarian mass, haptoglobin, CA125, tetrameric, lung, bladder cancer, leukemia, breast cancer, malignant lymphoma, urogenital tumors
Ovarian cancer is a lethal gynecologic malignancy with greater than 70% of women presenting with advanced stage disease. Despite new treatments, long term outcomes have not significantly changed in the past 30 years with the five-year overall survival remaining between 20% and 40% for stage III and IV disease. In contrast patients with stage I disease have a greater than 90% five-year overall survival. Detection of ovarian cancer at an early stage would likely have significant impact on mortality rate.1 Given our knowledge about the steep decrease in survival rates relative to the stage at which the disease is diagnosed, it is reasonable to suggest that early detection remains the most promising approach with which to improve the long-term survival of ovarian cancer patients. Therefore, considerable efforts have been focused on the identification of diagnostic biomarkers for early detection of ovarian cancer.2,3 CA125 has been used as a serum marker of ovarian cancer for monitoring responses to chemotherapy, detecting disease recurrence, distinguishing malignant from benign pelvic masses, and potentially improving the designs of clinical trial. However, CA125 has proven to be a poor diagnostic tumor biomarker for early stage ovarian cancer.4 It is elevated above reference levels in only 50% of clinically detectable early stage disease, and is not infrequently elevated in patients with benign ovarian tumors.5 Haptoglobin is an acute phase protein that binds hemoglobin, produced primarily by hepatocytes.6 The basic haptoglobin molecule is a tetrameric protein with α/β dimmers. Β chains are identical in all haptoglobin types and polymorphisms of haptoglobin rise from differences in α chain.7 In a manner similar to other acute-phase proteins, an elevation of this peptide could be observed in infections, inflammations, and various malignant diseases, including lung and bladder cancer, leukemia, breast cancer, malignant lymphoma and urogenital tumors.8,9
This study was carried out on 90 women attending El-Hussein and Bab El-Sheria University Hospital and outpatient Gynecological Clinic, a prospective study was held on patients with ovarian mass prepared for surgery. It is a case control study. It incorporated on 30 healthy women as a control group of which 24 pre menopausal, 6 postmenopausal and 60 patients with ovarian masses, of which 34 pre menopausal, 26 postmenopausal. Those patients were subjected to careful history taking, clinical examination, ultrasound examination and preoperative assessment of serum levels of Haptoglobin and CA125.
Statistical analysis of data:
The collected data was organized, tabulated and statistically analyzed using Statistical Package for Social Science (SPSS) version 20.0 running on IBM compatible computer with the help of Microsoft Office Excel 2007.
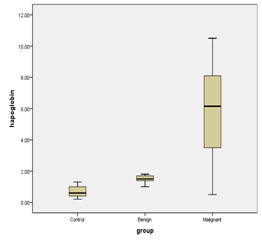
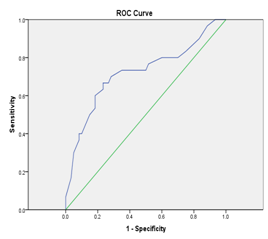
The tumor markers CA-125 and Haptoglobin were measured in the all studied patients (Table 1) (Figure 1). On analysis of serum CA-125, it was found that there is significant difference between the three studied groups with p value 0.0001 (Table 2) (Figure 2). On analysis of serum haptoglobin, it was found that there is significant difference between the three studied groups with p value 0.0001 (Table 3) (Figure 3). Analysis of CA-125 as a single biomarker using calculated cut off value > 36 U/ml shows that the sensitivity of CA-125 was 90.0%, specificity 92.0%, positive predictive value 88.0%, and negative predictive value 91.0% in the diagnosis of malignant ovarian tumors with p value = 0.002 (Table 4) (Figure 4). Analysis of Serum haptoglobin as a single biomarker using calculated cut off value > 1.9 mg /ml shows that the sensitivity of haptoglobin was 88.0%, specificity 85.0%, positive predictive value 84.0%, and negative predictive value 83.0% in the diagnosis of malignant ovarian tumors with p value = 0.0016 (Table 5) (Figure 5). Analysis of both Serum CA-125 and haptoglobin biomarkers using calculated cut off value > 36 U/ml and > 1.9 mg/ml respectively shows that the sensitivity of both was 94.0%, specificity 93.0%, positive predictive value 95.0%, and negative predictive value 92.0% in the diagnosis of malignant ovarian tumors with p value = 0.001.
Ca 125 (U/ml). |
Control Group No.= 30 |
Benign Group No.= 30 |
Malignant Group No.= 30 |
Range |
10.50 - 32.00 |
20.00 - 80.00 |
88.00 - 463.00 |
Mean |
18.75 |
50.37 |
231.93 |
S.D. |
5.79 |
13.49 |
115.17 |
F |
88.36 |
||
p |
0.0001** |
||
Table 1 Comparison between the three studied groups regarding serum Ca 125 (U/ml)
**Significant at level 0.01
Haptoglobin (mg/ml) |
Control Group No.= 30 |
Benign Group No.= 30 |
Malignant Group No.= 30 |
Range |
0.20 - 1.30 |
1.00 -1.80 |
0.50 -10.5 |
Mean |
0.67 |
1.5 |
5.94 |
S.D. |
0.33 |
0.22 |
2.77 |
F |
92.18 |
||
D |
0.0001** |
||
Table 2 Comparison between the three studied groups regarding serum haptoglobin (mg/ml)
**Significant at level 0.01
Variable |
Value |
Area under the ROC curve (AUC) |
0.69 |
Cut off value (U/ml) |
>36.0 |
Sensitivity % |
90 |
Specificity % |
92 |
PPV % |
88 |
NPV % |
91 |
P value |
0.002 |
Table 3 The sensitivity and specificity of Serum Ca 125 level to predict benign and malignant tumor using receiver -operating characteristics (ROC) curve with cut off value >36 U/ml
Variable |
Value |
Area under the ROC curve (AUC) |
0.62 |
Cut off value (mg/ml) |
>1.9 |
Sensitivity % |
88 |
Specificity % |
85 |
PPV % |
84 |
NPV % |
83 |
P value |
0.016** |
Table 4 The sensitivity and specificity of Serum haptoglobin level to predict benign and malignant tumor using receiver -operating characteristics (ROC) curve with cut off value >1.9 mg/ml
**Significant at level 0.01
Variable |
Value |
Area under the ROC curve (AUC) |
0.81 |
Sensitivity % |
94 |
Specificity % |
93 |
PPV % |
95 |
NPV % |
92 |
P value |
0.001 |
Table 5 The sensitivity and specificity of combined use of Serum Ca 125 and haptoglobin level to predict benign and malignant tumor using receiver - operating characteristics (ROC) curve with cut off value >36 U/ml and >1.9 mg/ml respectively
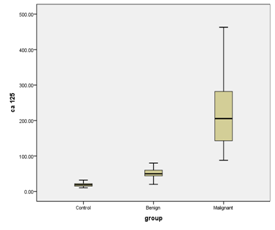
In the present study, CA-125 at a cut off 36 U/ml was a significant predictor of malignancy in the studied cases. The result is in agreement with Ong et al.10 who found significant differences between median CA-125 levels between benign and malignant ovarian masses. In agreement with Ye at al.11 who found that mean serum CA-125 was significantly higher in patients with cancer ovary compared to normal subjects, patients with benign tumors and patients with other gynecological cancers. Ahmed et al.12 observed that the mean serum CA-125 in stage III ovarian cancer patients was significantly higher than in the control women (p< 0.05). Similarly, Dobryszcha et al.13 who observed that serum CA-125 levels were significantly higher in malignant ovarian tumors Compared to non malignant ovarian tumors and normal subjects and it was also significantly higher in non malignant ovarian tumors compared to normal subject the initial findings of serum CA-125 levels greater than 35U/ml in approximately 83% of patients with epithelial ovarian cancer lead to use of serum CA-125 as a biomarker for ovarian cancer.14,15 In the current study, at cut off value of 36U/ml the predictive value of serum CA-125 in diagnosis of malignant ovarian tumors were as follow: sensitivity was (90%), specificity was (92%), and positive predictive value (88%) and negative predictive value (91%). These figures are close to Ye et al. (2003) they found that at cut off of 35 U/ml CA-125 had a sensitivity of 84% but low specificity 35.2%. Also, Gadducci et al.16 reported that diagnostic value of serum CA125 in distinguishing a benign from malignant ovarian mass has been demonstrated with a sensitivity ranging from 56%-100% and a specificity ranging from 60%-92%. In other study Su et al.17 they found that the evaluation of serum CA-125 as a marker in detection of ovarian carcinoma was: sensitivity of 50-62%, specificity of 30%, positive predictive value of 57% and negative predictive value of 70.6%. The low levels in these studies were explained by choosing patients with early stage ovarian carcinoma. In the current study, the serum CA-125 ranged from (88-463) with a mean 231.93 in malignant group. It was significantly lower in benign compared to malignant group (p=0.0001).
Ahmed et al.12 observed that the mean serum haptoglobin expression in stage III ovarian cancer patients was four fold higher than in the control subjects (p< 0.05). They carried their study on women with high grade ovarian cancer and compared to a control group of healthy women. Similarly, Ye et al.11 studied 80 cancer patients. They found that serum haptoglobin was significantly higher in cancer ovary. Also, Zhao et al.18 observed that mean serum haptoglobin was significantly higher in malignant ovarian tumors even those with early stage tumor, compared to those with benign conditions. The same results were obtained by Dobryszycka et al.13 who studied serum haptoglobin levels in malignant and non malignant tumors of the ovary, they found that serum haptoglobin levels was significantly higher in malignant ovarian tumors compared to non-malignant tumors and normal subjects. This indicated that haptoglobin plays an important role in the evaluation of the cancer ovary. Other investigations revealed that haptoglobin is natural inhibitor of collagen degradation and is locally expressed by fibroblasts in arterial walls.19 This glycoprotein therefore plays an important role in cell migration and in arterial destructing. Moreover, collagen turnover is an important feature in many physiological processes such as growth and wound healing, and enhanced collagen degradation is causally related to severe tissue destruction and malfunction which are often encountered in pathological processes such as arthritis and cancer.20 The importance of haptoglobin in extra cellular matrix degradation and cell migration suggests a role for this polypeptide in cancer and also provide evidence supporting haptoglobin as a molecular target for therapy in epithelial ovarian carcinoma.12
Chen et al.15 demonstrated that the serum and tissue haptoglobin were significantly higher in patients with epithelial cell ovarian carcinoma and germinal cell ovarian carcinoma compared to the control normal subjects. In the current study, at cut off value of 1.9 mg/ml the accuracy value of serum haptoglobin in diagnosis of malignant ovarian tumors is: sensitivity was (88%), specificity (85%), positive predictive value (84%) and negative predictive value (83%). In the current study the serum haptoglobin ranged from (0.5-10.5) mg/ml with a mean of 5.94 in malignant group, it is significantly different from control group (P<0.0001). The serum haptoglobin in benign group ranged from (1-1.8) mg/ml with a mean 1.50 which is significantly different from than control group (P< 0.0001). It was found that serum level of haptoglobin in malignant and benign group was significantly different from control group (P< 0.0001), while serum level of haptoglobin in malignant cases are more than that in the benign cases. These results were in agreement with Ye et al.11 who found that there are 80 patients with ovarian cancer, 51 patients with benign ovarian tumor, 120 normal controls and 52 other gynecological cancers. The results in malignant cases were significant difference (p< 0.001) from normal controls, benign ovarian tumors and other gynecological cancers respectively and agreed with Zhao et al18 who found that 66 malignant patients, 60 benign cases and healthy controls, he observed that haptoglobin concentration were significantly higher in late stage ovarian cancer than benign and healthy controls (P< 0.05 and P< 0.01) and there was significant difference between early stage cancer and healthy controls (P< 0.01).
This in agreement with Katnik et al.21 who found that in the group of ovarian cancer haptoglobin level was more than three times than control groups, the group of non malignant tumors the haptoglobin level was twice as high as in the control group. The combined ability of both markers (serum CA-125 and serum haptoglobin) to detect ovarian malignant tumors was tested in the current study at the cut off value of 36 U/ml for serum CA-125 and 1.9 mg/ml for serum haptoglobin, the predictive values of both marker together in diagnosis of malignant ovarian tumor were as follow: sensitivity was (94%), specificity was (93%), positive predictive value was (95%) and negative predictive value was (92%). The results of this study are similar to that of Ye et al. (2003) they found that the combined ability of both markers (serum CA-125 and serum haptoglobin) showed better results with 91% sensitivity and 95% specificity in the whole study groups.
None.
The author declares no conflict of interest.

©2016 Khalaf, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.