MOJ
eISSN: 2475-5494


Research Article Volume 8 Issue 2
1Department Obstetrics & Gynecolgy, University of Kuwait, Kuwait
22Department Obstetrics & Gynecolgy, Adan Hospital, Kuwait
Correspondence: Baydaa Al Sannan, Department of Obstetrics and Gynecology, Faculty of medicine, Kuwait University, P.O. BOX 24923, SAFAT 13110, Kuwait, Tel +965 25319601, Fax 965-25338904
Received: December 18, 2018 | Published: March 5, 2019
Citation: Sannan BA, Harmi JA, Nandakumaran M, et al. Comparative assessment of pregnancy outcome in diabetic obstetric population in Kuwait tertiary hospital. MOJ Womens Health. 2019;8(2):126-131. DOI: 10.15406/mojwh.2019.08.00224
Objective: Although diabetes mellitus is known to complicate pregnancy, data on pregnancy outcome in diabetic pregnancies are scanty. Hence we decided to conduct a retrospective study on pregnancy outcome in pregnancies complicated by Type2 diabetes, in an obstetric population in a Kuwait tertiary hospital.
Material and Methods: A total of 1250 women were scanned to evaluate pregnancy outcome in Type2-Diabetic and control pregnancies. In control group, 125 pregnant women were enrolled with inclusion criteria of singleton delivery between 35-41 weeks of gestation and having uncomplicated course of pregnancy. Only those clinically diagnosed with Type 2 Diabetes (n=20), were included in the Study. Statistical significance between obstetric and control groups was assessed using the SPSS software.
Results: Maternal weight at the time of delivery, blood sugar level, HbA1C level, placental weight, mode of delivery and newborn: placental ratio were significantly higher (Student’s t-test, p<0.05) in Diabetic Population (n=20) compared to control population (n=125). However, Student’s t-test showed no significant difference (p>0.05) between gestational age at delivery, and weight of newborn at delivery. Neonatal weight: placental weight ratio (BPR) in diabetic group averaged 5.30±0.15 while in the control group the ratio averaged 4.95±0.22, Student’s t-test showed no significant difference in BPR values in the 2 Groups.
Conclusion: Type2 Diabetes in Pregnancy was not associated with significantly BPR value in diabetic pregnancies compared to control population Absence of significant increase in birth weight of newborns in diabetic population could be attributed to the high quality obstetric and clinical care in Kuwait.
Keywords: pregnancy, outcome, type2, diabetic, pregnancies, newborn, weight, placental, ratio
Though incidence of Diabetes Mellitus in Pregnancy is a serious complication of Pregnancy, data on pregnancy outcome in diabetic pregnancies in Kuwaiti Obstetric Population are scanty. Hence we have attempted to establish baseline parameters of Pregnancy outcome in Type 2 Diabetic Pregnancies including Gestational Diabetic Pregnancies by conducting a retrospective survey of Type 2 Diabetic Pregnancies and compared the Date Obtained from control, uncomplicated normal pregnancies in a Tertiary Hospital in Kuwait.
Assessment of neonatal birth weight and placental weight are considered crucial pre-requisites to assess pregnancy outcomes in all developed countries. The pattern of fetal growth and the eventual birth weight are determined by genetic and environmental factors. Both impaired fetal growth and augmented fetal growth have been associated with increased mortality or morbidity that appears to be related more often to the cause of the aberrant growth than to size it.1 An increased risk of death in association with impaired fetal growth resulting from chronic utero-placental insufficiency is well known, and the risk to the fetus appears to increase as the birth weight percentile drops. Fetal and neonatal mortality is also increased with other specific causes of impaired fetal growth, such as aneuploidy or embryonic infection.1 Birth trauma-associated with augmented fetal growth is also well known and has been associated either with birth weight >4000 g or>4500 g or with birth weight >90th percentile. However, the risk of death associated with augmented fetal growth has only rarely been examined.2 The newborn weight: placental weight (NBPW) ratio is a measure of the balance between fetal and placental growth. This ratio increases during gestation as the fetus matures concurrent with placental growth and increased transport capacity with corresponding increase in fetal weight. Many reports have established that placental weight and the birth weight and newborn weight: placental weight ratios are predictive of maternal disease, obstetric outcome, perinatal morbidity and mortality, and childhood growth as well as intra-uterine growth and development.3–5
Augmented fetal growth defined by use of absolute weight criteria ignores the possibility that pathologic augmented growth may be seen at earlier gestational ages, when equally aberrant growth may not result in birth weight change beyond reported weight thresholds. Furthermore, absolute weight criteria, regardless of the pattern or the clinical context of the augmented growth, ignore specific risks that may be related to specific clinical circumstances. Through placental weight is generally lower SGA (small for gestational age) infants,6–8 this correlation does not reflect the compromised placental function and adaptive hypertrophy and increased weight of placentae in conditions such as maternal malnutrition’s, etc.9
Variations in birth weight standards, both regional and national and international and the variations in these standards have led to the recommendation that local or regional birth weight standards be used for the diagnosis of anomalous growth.1,2 Diabetes Mellitus is well documented to be associated with increased maternal and neonatal mortality and morbidity. Considering the paucity of data relating to pregnancy outcome in Type2 Diabetic Pregnancies, we decided to undertake a retrospective study of diabetic pregnancies population in a Tertiary Hospital in Kuwait and to compare the pregnancy outcome data with control uncomplicated pregnancies. We firmly believe that establishing baseline pregnancy outcome parameters are crucial in evaluating factors that could adversely affect the health of the mother and the baby in conditions such as diabetes mellitus in pregnancy.
The study was a cross-sectional study between first of October 2013 to end of March 2014 at Adan Hospital, Kuwait, serving an obstetric population of about 70000 women per year. The Hospital has a 500-bed space with annual delivery rate of 7000. One thousand and two hundred women were scanned for the study. The selection criteria were singleton delivery between 36-41 weeks of Kuwaiti obstetric population. The exclusion criteria included retained placenta, multiple pregnancies, adherent placenta, placental abruption and renal or liver and systemic diseases. In the case of pregnancies clinically identified as Diabetic Pregnancies, Only those diagnosed as Type 2 Diabetes using standard clinical and laboratory were included in the Study. Data on gestational age at delivery (in weeks), maternal age, parity, mode of delivery, birth weight, freshly delivered untrimmed placental weight, fetal gender and presence/absence of maternal medical diseases (e.g., hypertensive disorders and diabetes mellitus were collected from the Kuwaiti obstetric population. Gestational age of women was estimated using last menstrual period (LMP). However when the LMP was not known, the gestational age was computed using early ultrasound. All placentas were weighed shortly after delivery on table top weighing scale together with the membranes and the cord after removing visible blood clots. The placental: birth weight ratio (PBR) of control and diabetic patients was calculated as ratio of placental weight to neonatal weight. The weights of the newborn babies were recorded to the nearest gram. Measurements of newborn weights were made by the nursing staff on duty or the duty doctor using the same table top weighing scale. Data were processed using the SPSS software and statistical analysis performed using one-way analysis of variance (ANOVA) or Student's t-test as appropriate. A probability limit of 0.05 was set to determine statistical significance.
Clinical Characteristics of Control and Diabetic Population are shown in Table 1. Unpaired Student’s t -test showed that weight of mother at delivery, blood sugar and HbA1C Levels, previous abortion rate, placental weight newborn weight: placental weight ratio were significantly higher ( p<0.05) in the diabetic group that those of control pregnant group. However no significant difference (p>0.05) was observed in the mean gestational age at delivery and birth weight of newborns in the diabetic group compared to control group. Weights of newborn and placentae in control population as a function of gestational age at delivery are detailed in Table 2. Birth weight and placental weight as well as NB: PB Ratio at 35th Week of Delivery were significantly lower (Student’s t-test; p<0.05) than those of other weeks. Table 3 details weight of newborn, weight of placenta and NB: PB Ratio corresponding to various gestation ages at delivery in diabetic pregnant women. Placental weight and newborn weight at 35th week and 36th Week of Delivery and NB: PB ratio was significantly lower compared to the Corresponding values of 37, 38, 39, 40, 41 gestational weeks. However the surprisingly higher placental weight relative to newborn weight in the 37th week contributed to a significantly lower NB: PB Ratio (Student’s t-test p<0.05) in diabetic women compared to corresponding NB: PB ratio observed in other clusters. Placental weight: Birth Weight Ratio (PWR) for 35, 36,37,38,39, 40 and 41 weeks of gestation averaged 0.22, 0.22, 0.24, 0.19, 0.17, 0.19 and 0.20 in the Diabetic Study Group while the corresponding PWR Values for control group averaged 0.22, 0.22, 0.20. 0.18, 0.20, 0.18 and 0.17 respectively. ANOVA Test did not show any statistical significance (p>0.05) between control study group PWR Values.
Characteristics |
Control |
Diabetic |
No. of patients (n) |
125 |
20 |
Mother's weight (Kg) |
66.4 |
73.6* |
Blood sugar (mmol/L) |
4.5 |
5.2* |
Age (Years) |
28.33 |
33.2* |
Previous abortion (%) |
20 |
35* |
Mode of delivery (CS)(%) |
25 |
45* |
Gestational age (Weeks) |
38 |
37 (NS) |
HbA1C (%) |
5.79 |
6.95* |
Birth weight (g) |
3006.83 |
2960.85 (NS) |
Placental weight(g) |
569.76 |
616.5* |
NB: PB Ratio |
5.3 |
4.96* |
Table 1 clinical characteristic of Control and Diabetic Patients
*Values are expressed as Means of each item in each group statistical significance was assessed using unpaired students t-test (*p<0.05).
*NS, Not significant.
Gestational age(Weeks) |
Birth Weight(gm) |
Placental Weight(gm) |
NB:PB Ratio |
35 (6)* |
2268.33±133.9* |
493.33±19.2* |
4.53±0.14* |
36 (8) |
2533±192.3 |
556.25±21.5 |
4.71±0.36 |
37 (14) |
2683.14 ±85.3* |
531.43±9.7 |
5.02 ±0.152* |
38 (23) |
2894.77±86.4* |
582.17±21* |
5±0.17* |
39 (22) |
3155.91±97.6* |
580.45 ± 7.9* |
5.5 ±0.18* |
40 (40) |
3186.75±60.9* |
579.0 ±6.8* |
5.5 ±0.09* |
41(11) |
3406.6 ±100.3* |
589.09 ±15.04* |
5.88±0.14* |
Mean±SEM |
3006.83±42.63 |
569.76±5.35 |
5.3±0.068 |
Table 2 Weight of newborn and placenta in Control Population (n=125)
*Numbers in Brackets indicate number of patients in each group.
*NB, New born Weight; PB, Placental weight
*Students t-test (p<0.05)
Gestational age (Weeks) |
Birth Weight (gm) |
Placental Weight ( gm) |
NB:PB Ratio |
35 (1)* |
2190 |
480.00* |
4.56 |
36 (1) |
1890.00* |
410.00* |
4.61 |
37 (9) |
2824.11±172.4* |
682.2±69.4 |
4.36±0.32* |
38 ( 1) |
3020.00* |
580 |
5 |
39 (4) |
3442.5±196.9* |
592.5 ±9.46 |
5.97±0.39* |
40 (1) |
2850.00* |
530 |
5.38* |
41(1) |
3270.00* |
640 |
5.11* |
2960.85 |
616.5 |
4.96 |
|
Mean±SEM |
2960.85±129.142 |
616.50±12.840 |
4.96±0.225 |
Table 3 Weight of newborn and placenta in diabetic Population (n= 20)
*Numbers in Brackets indicate number of patients in each group.
*NB, New born Weight; PB, Placental weight
*Students t-test (p<0.05)
Figure 1 shows the relation of birth weight as a function of gestational age in control and diabetic group. Analysis of c-variance did not reveal any significant Difference Between the Control and Diabetic Groups( p>0.05) though the growth curve was apparently more erratic in the case of diabetic group compared to control Group. Figure 2 shows the relation of placental weight as function of gestational age in diabetic pregnant women. Analysis of c-variance did not reveal any significant Difference between the Control and Diabetic Groups (p>0.05) though the placental growth curve was apparently more erratic in the case of diabetic group compared to control Group. Relation of NB: PB ratio as a function of gestation age is shown in Figure 3. Analysis of c-variance did not reveal any significant Difference in NB: PB Ratio between the Control and Diabetic Groups (p>0.05) though the ratio was apparently more dispersed in the case of diabetic group compared to control Group.
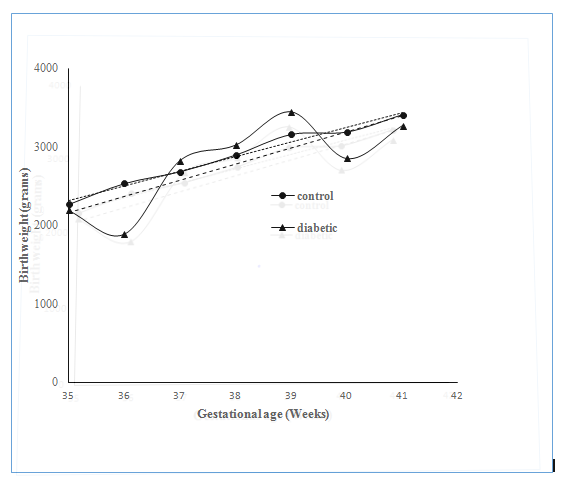
Figure 1 Relationship of Birth Weight as Function of Gestational Age at Delivery in Control and Diabetic Groups.
Regression Line for the Control Group was Drawn using the following equation: y = 185.54x – 4175 While the Regression Line for the Diabetic Group was using the Following equation: y = 206.37x – 5058.3 Statistical Significance was assessed using ANCOVA Test (p>0.05).
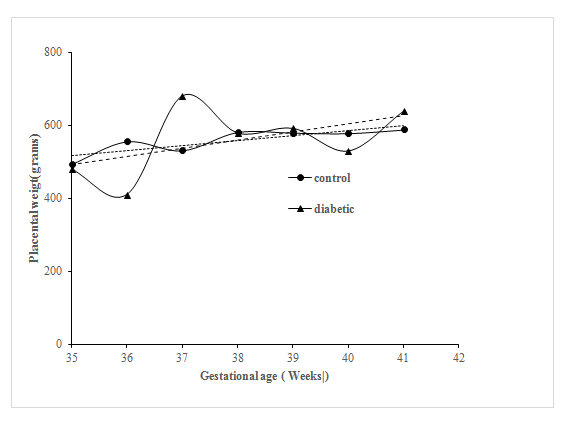
Figure 2 Relationship of Placental Weight as a Function of Gestational Age at Delivery in Control and Diabetic Groups.
Regression Line for the Control Group was Drawn using the following equation: y = 13.636x 40.66 While the Regression Line for the Diabetic Group was using the Following equation: y = 22.511x–296.16 Statistical Significance was assessed using ANCOVA Test (p>0.05).
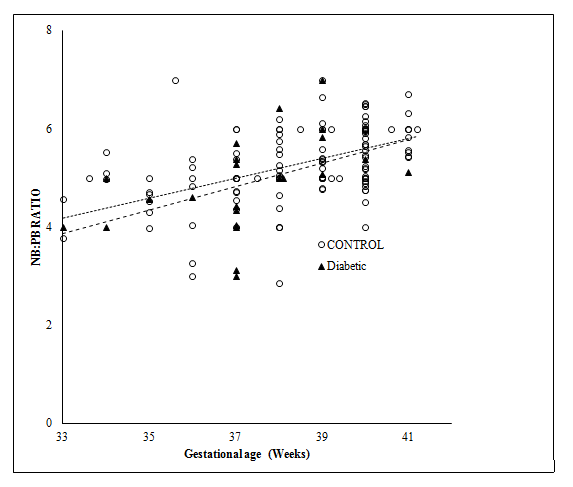
Figure 3 Relationship of Birth Weight; Placental Weight (NB: PB) Ratio as Function of Gestational Age at Delivery in Control and Diabetic Groups.
Regression Line for the Control Group was Drawn using the following equation: y = 0.2027x –2.5187 While the Regression Line for the Diabetic Group was using the Following equation: y = 0.2398 x–4.0457 Statistical Significance was assessed using ANCOVA Test (p>0.05).
This survey was meant mainly to establish reference criteria for assessing pregnancy outcome in control uncomplicated pregnancies as well as in pregnancies complicated by diabetes mellitus. Due to strict application of exclusion and inclusion criteria, the number of diabetic patients was less compared to control pregnancies surveyed during the period of study. Placental weight: neonatal weight ratio (PWR) is well recognized to be a health indicator for assessing fetal and placental growth. PWR ratios calculated in this study are comparable to those reported by various research groups.3,8,10,11 However considering the greater spread of the of NB:PW Ratios, when compared to the corresponding PWR Ratio Values, we believe that NB:PW ratio and absolute weights of newborn and placentae are better indicators or pregnancy outcome than the latter. It is worth highlighting the fact that though fetal and placental growth is conventionally assessed using feto-placental ratio or birth weight to placental ratio, that measure is problematic because a “normal ratio” can be seen when both newborn weights and placental weights are normal, or low or both the weights are high to12 and hence absolute birth weight and placental weight values are better indictors of fetal growth and placental growth per se. It is well established that there is increased risk of stillbirth with low placental weight supporting the hypothesis that reduced placental surface area for nutrient and gas exchange could lead to compromised fetal development.13–15 All the same, increased placental weight per se can’t be taken as an indicator for increased placental functional efficiency as well. Incidence of increased placental weight associated with adverse neonatal outcome could be a reflection of the compensatory adaptation of placental tissue and this could explain the relatively larger weights of placenta at birth at high altitudes or in pregnancies complicated by factors such as maternal anemia, smoking in pregnancy, etc.16–19 More detailed investigation and surveys are warrant ed to assess the suitability of either of the ratios as reflectors of fetal growth and placental growth though possibility of placental hyperplasia as a compensatory adaptation to counter fetal distress cannot be overlooked. It is likely that the relatively higher weights of placentae in diabetic women have led to significantly lower NB: PB ratio observed in this study. This is the first ever reported study on assessment of pregnancy outcome in diabetic pregnancies as well as control pregnancies in Kuwait and could serve as baseline data for assessing pregnancy outcome in control and diabetic pregnancies though it is impossible to predict whether data reported in this retrospective survey could be extrapolated to diabetic pregnancies complicated by other organ dysfunctions or to insulin-dependent or Type 1 Diabetic Pregnancy situations requiring varying doses of insulin to counter varying grades of hyperglycemia or in pregnancies complicated with ketonuria and other metabolic dysfunctions. The data on weights of newborns and placental weight in control obstetric population are comparable to those reported by several investigators.20–22
It’s pertinent to note that majority of placental growth occurs before 33rd week of gestation23and hence this study focusing on deliveries between gestational ages ranging from 35-41 weeks may not adequately reflect the possible changes or disturbances of placental development in pregnancies complicated by diabetes mellitus compared to control pregnancies. It is unclear whether altered metabolic environment of the diabetic mother would have been responsible for the wavy pattern of fetal birth weight and placental weight curve observed in our study of diabetic group compared to control pregnant women, surveyed for the period between 35 to 41 weeks of gestation. Interestingly no significant difference in birth weight was detected between control and diabetic groups and this finding is comparable to findings of Alfadhli etc. al surveyed in an obstetric population in Saudi Arabia.20 We are unable to explain the significantly lower placental weight and lower birth weight observed in women delivered at 36th week of gestation compared to the 37th week and recommend that, if the clinical condition of the mother and fetus permit, the delivery should be delayed to 37th week in diabetic pregnancies in order to avoid unhealthy neonatal squeal for the baby. Interestingly no such aberration inplacental weight and newborn weight was observed between 36th week and 37th week in the case of control pregnancies. We believe that better pregnancy outcome reported in the obstetric diabetic population is attributable to better antenatal care provided to diabetic patients in our hospital and better clinical monitoring and stricter control of hyperglycemia using appropriate drugand diet regimen in affected patients. We are currently investigating whether pregnancy outcome differs in pregnancies complicated by Type 1 Diabetes in Pregnancy and extending the present study using a larger population size as well.
This Retrospective survey done in tertiary Hospital showed that in pregnancies complicated by Type2 Diabetic Pregnancies, there was no significant difference between newborn weights: placental weight ratio compared to control uncomplicated normal pregnancies although placental weight in the diabetic group was significantly higher than that of control group. We believe that the control of weight of newborns on diabetic pregnancies to be within the range observed in control uncomplicated pregnancies is due to high quality obstetric and clinical care being given to diabetic patients in the Hospital concerned. We are currently investigating whether pregnancy outcome differs in pregnancies complicated by Type 1 Diabetes in pregnancy and extending the present study to a larger population size as well.
None.
The author declares there are no conflicts of interest.

©2019 Sannan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.