MOJ
eISSN: 2475-5494


Review Article Volume 8 Issue 3
1Department of Obstetrics and Gynaecology, Benha University, Benha, Egypt
2Department of Obstetrics and Gynaecology, October 6 University, Giza, Egypt
3Department of Obstetrics and Gynecology, Armed Forces Hospital Southern Region, Saudia Arabia
Correspondence: Ahmed Altraigey MD, Department of Obstetrics and Gynaecology, Benha University, 43 benha zagzig street, Mansheyet Elnoor, Benha, Egypt, Tel +966544854232
Received: May 02, 2019 | Published: May 29, 2019
Citation: Altraigey A, Gamal A, Mostafa ST. Cesarean scar pregnancy: tertiary-centre experience. MOJ Women's Health. 2019;8(3):216-220. DOI: 10.15406/mojwh.2019.08.00239
Background: Past history of previous Cesarean delivery [CD] carries the risk for gestational sac implantation of any subsequent pregnancy over the cesarean scar developing what is defined as a cesarean scar pregnancy [CSP]. The aim of this article was to evaluate possible interventions used for the diagnosis and treatment of CSP.
Materials and methods: This cohort analysis of case series included all patients admitted to Obstetrics and Gynecology department, King Faisal Military Hospital over a period of 5 years [2014 through 2019 and evaluated in 2019] with the diagnosis of suspected CSP. Demographic characteristics, clinical and outcome data were obtained from the original electronic hospital charts, operation notes, anesthesia notes, discharge summaries, nursing notes and outpatient medical records.
Results: During the study period 12 patients were diagnosed by US as having CSP, the mean age was 36 years, the mean of parity was 4.7. Most of the patients had previous two CD [33%]. The mean time interval between the previous CD and the current CSP was 16.4 months and the estimated gestational age was 46 days. The mean diameter of the mass in the sac of CSP was 9.7 mm, and the mean of serum β-hCG levels was 53765.8 mIU/mL. All patients were successfully treated by methotrexate and the mean resolution time was 52 days.
Conclusion: Methotrexate [MTX] was effective for treatment of CSP but larger multicenter studies with large number of patients are necessary to confirm our results.
The Cesarean delivery [CD] rate increased markedly in the past two decades. Its rate was doubled between 2000 and 2015 to reach almost 21% of all live births. This increase was noticed in 169 countries that reported 29.7 million deliveries by CD annually.1 This raise could be explained by the rise of primary [first] CD [from 12.6-20.6%] and a decline in vaginal births after CD [28-9.2%], so that the rate of repeat CD is now about 91%.2 The maternal morbidity prevalence is higher after CD than after normal vaginal birth. CD is associated with a higher incidence of ectopic pregnancy, abnormal placentation [placenta previa\accreta] and uterine rupture. Moreover, these risks increased in a dose–response manner.3 History of a past CD increased the risk of gestational sac implantation of the next pregnancy over the cesarean scar, creating the clinical condition defined as cesarean scar pregnancy [CSP] and as explained the magnitude of this risk raised with more repeated CD.4 Two types of CSP were recognized according to the extent of gestational sac invasion; Type 1: where superficial implantation on a scar progressing subsequently towards the cervico-isthmic space and/ or the uterine cavity, whereas Type 2: direct deep implantation into the myometrium±reaching up to inner surface of uterine visceral serosa.5 Since 2000, CSP incidence showed a significant increase, up to 6.1% of all ectopic pregnancies in women with past history of CD, which might be attributed to both increased number of CD and the improved diagnostic accuracy tools recognizing ectopic pregnancy.6 CSP carried the risk of major bleeding, fatal hemorrhage, and spontaneous uterine rupture up to hysterectomy to save the women’s lives.7 Although its clinical presentation vary between light painless vaginal bleeding and moderate abdominal pain, the accurate diagnosis remained difficult as the false negative results of multiple tests could lead to a life-threatening scenario.8 The ideal pathway of CSP management is widely controversial. However, it is universally agreed that surgery is the unavoidable 1st line of management of women presenting with uterine rupture or severe bleeding. On the other hand, the management of hemodynamically stable diagnosed with CSP might represent a challenge. There were almost 31 primary approaches published to treat CSPs but mostly sporadic and individual cases and their results seemed to be insufficient to conclude clearly which one was the most effective management protocol that leaded to the least adverse events. Thus, there is increased needs to develop a set of practice guidelines for health care professionals considering optimum management of CSP. The aim of this work was to describe the experience of a tertiary care hospital with the diagnosis and treatment of CSP and to explore patients' complications related to this rare type of ectopic pregnancy.
This Cohort study was conducted in King Faisal Military Hospital, Khamis Mushayt, Southern Region, Saudi Arabia in the period from June 2015 to January 2019 after obtaining the approval of the local institutional ethics and research committee [Armed Forces Hospital Southern Region ethics and research committee]. All patients admitted to Obstetrics and Gynecology Department, King Faisal military hospital over a period of 5 years [2015-2019] with the diagnosis of suspected CSP were included. Hemodynamic instability that required immediate surgical intervention was the only exclusion criteria. All patients were consented before starting data collection and after full counselling about the hysterectomy risk. All patients accepted the conservative treatment including methotrexate injection, D&C, or laparotomy and wedge resection of the scar ectopic pregnancy because they all asked for fertility preservation. When CSP was suspected by a thorough history taking, including obstetric, reproductive, surgical history as well as physical examination, the diagnosis was confirmed by the following US findings: (Figure 1)
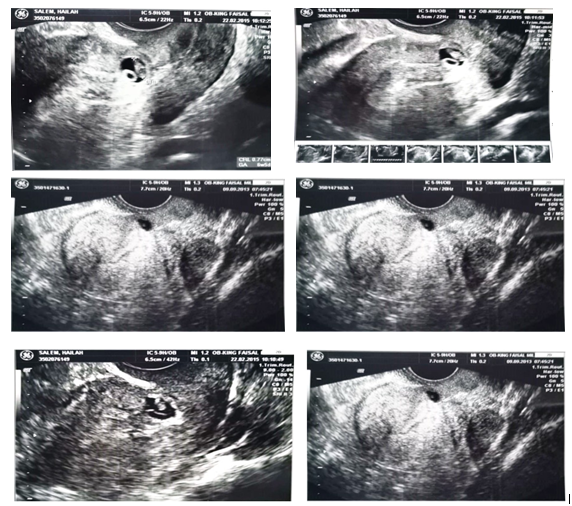
Figure 1 Ultrasound findings conclusive of CSP; mainly the gestational sac is embedded inside myometrium and empty uterine cavity.
Data were abstracted from the original hospital charts, operation notes, anesthesia notes, discharge summaries, nursing notes and outpatient medical records. From each patient’s file, the following data were searched for age, parity, presenting symptoms, number of previous CD, duration from last cesarean section, gestational age at diagnosis, fetal cardiac activity, initial diagnosis [e.g. early pregnancy, cervical pregnancy, incomplete miscarriage], Β-hCG concentration [at admission and then at different times of follow-up], management plan, complications [whether or not related to the management] and time interval needed for β-hCG to return back to normal. Analysis of the data was done by IBM computer using SPSS [statistical product and service solution version 21]. Quantitative variables were described as mean with range while qualitative variables will be described as numbers and percentages.
During the study period 12 patients were diagnosed by US as having CSP, the range of the age was between 32 and 45 years old. The lowest parity was 1 and the highest was 10. The mean [±SD] of body mass index [BMI] was 30.8±4.4 kg/m2. The interval between recent pregnancy and the last delivery ranged from 10 months to 24 months. It needed between 33 days and 77 days gestational age to diagnose CSP. All women got pregnant spontaneously and 33% of them had previous two CD as the highest frequency as shown in Table 1. 58.3% women experienced abdominal pain, while 33% were identified asymptomatically as an incidental finding. The mean [±SD] of serum pretreatment β-hCG levels and hemoglobin were [20432.5±21082.7 mIU/mL, 11.9±1.5 gm/dl respectively]. All women were diagnosed solely using ultrasound [US] without any need for extra imaging tools. No women were diagnosed with intra-peritoneal fluid. Five women [42%] showed signs of fetal cardiac activity Table 2. The whole cohort received methotrexate therapy as the 1st line treatment which was successful initially in seven women [58.3%], while the rest needed extra course of same medication. It needed around 300 mg and 58 days of methotrexate to achieve effective decline of β-hCG Table 3. Only one patient on the 5th day following Methotrexate [MTX] injection suffered from excessive vaginal bleeding and surgical interference was mandatory to save the patients life, so emergency suction curettage under US guidance was performed. All patients showed no drug side effects.
Variable |
Mean±SD |
|
Women age [years] |
36.1±4.1 |
|
Parity |
4.7±2.6 |
|
BMI [kg/m2] |
30.8±4.4 |
|
Interval from last pregnancy [months] |
16.4±5.7 |
|
Gestational age at diagnosis [days] |
46.0±12.3 |
|
Variable |
Frequency [%] |
|
Spontaneous |
12 [100%] |
|
Mode of conception |
Assisted |
0 [0] |
Number of previous CD |
One |
3 [25%] |
Two |
4 [33%] |
|
Three |
3 [25%] |
|
Four |
2 [17%] |
|
Table 1 Characteristics of women under the study
SD, Standard Deviation
Variable |
Mean±SD |
|
β-hCG at admission |
20432.5±21082.7 |
|
Hemoglobin at admission [gm/dl] |
11.9±1.5 |
|
Size of the mass [mm] |
9.7±4.2 |
|
Variable |
Frequency [%] |
|
Abdominal pain |
7 [58.3%] |
|
Clinical presentation |
Vaginal bleeding |
1 [8.3%] |
Asymptomatic |
4 [33.3%] |
|
Presence of fetal cardiac pulsations |
Present |
5 [41.7%] |
Absent |
7 [58.3%] |
|
Presence of intra-peritoneal hemorrhage |
Present |
0 [0] |
Absent |
12 [100%] |
|
Table 2 Clinical characteristics of women under the study
SD, Standard Deviation
Variable |
Mean±SD |
|
Total dose of MTX given [mg] |
350±172.9 |
|
Time to normalization of β-hCG [days] |
52.5±12.9 |
|
Variable |
Frequency [%] |
|
Number of methotrexate courses |
a) One course |
7 [58.3%] |
b) 2 course |
5 [41.7%] |
|
Complications |
a) Present |
1 [8.3%] |
b) Absent |
11 [91.7%] |
|
Table 3 Outcome measures of women under the study
SD, Standard Deviation
Cesarean scar pregnancy is considered as a rare form of ectopic pregnancy, and its incidence in our hospital may be increased due to the rise of the rate of Cesarean sections performed. The incidence of Cesarean scar pregnancy was 1: 2216 [12 cases/26,596 women at the study period] in this study and its rate was 6.1% [12 scar pregnancies/198 ectopic pregnancies] with a history of at least one previous Cesarean section. The incidence of Cesarean scar pregnancy was 0.15% [12 cases/7980 CD] in women with previous CD. There are two explanation of this rise in our hospital. Firstly, being a tertiary referral center of a local district where many women suffering abnormal or ectopic pregnancies were referred for management. Second, the higher frequencies the transvaginal US provided allowed early diagnosis of abnormal pregnancies. Increased recognition and the number of CD are presumably responsible for that rise in numbers of CSP. The natural history of CSP condition is unknown, but spontaneous scar rupture and hemorrhage, even early as in the first trimester, if the pregnancy is permitted to resume, with potential dangerous maternal adverse events. Myometrial and endometrial scarring or disruption might lead to implantation abnormality. Also, trophoblast invasion or adherence increased due to the scanty decidualization of the lower uterine segment. However, pregnancy implantation within the scar of a previous CD differed from pregnancy with placenta accreta. Implantation site of CSP is completely surrounded by myometrium and the scar fibrous tissue and totally separated from empty endometrial cavity. The mechanism that mostly could explain implantation within the fibrous scar is myometrial invasion through a microscopic tract. This tract is thought to be due to the surgical trauma of previous uterine procedure like curettage, manual removal of the placenta, CD, myomectomy and even hysteroscopy.9 The time elapsed between trauma and the following pregnancy had an impact on implantation as some reported cases were detected within months of a prior CD which suggested that if uterine scar was incompletely healed, it contributed to scar implantation.10 Veridiano 11 described a case complicated by uterine perforation during curettage at 14 weeks of gestation due to placenta percreta in a uterine scar. An emergency hysterectomy was performed following profuse bleeding. Herman12 described the first case detected using US that revealed an early gestational sac implanted inside a uterine scar. Their case was allowed to follow up her pregnancy that ended by emergency CD and hysterectomy due to severe bleeding that preceded intravascular coagulopathy. Early diagnosis is critical for offering multiple treatment options so that young women who desire to preserve fertility can be managed conservatively. Although diagnosis of ectopic pregnancy by US was reported more than four decades ago, the use of US in detecting a CSP was not reported until 1990.13 Strict criteria in US imaging must be used to differentiate CSP from spontaneous abortion in progress, cervico-isthmican empty uterine cavity, an empty cervical canal, gestational sac located in the anterior part of the uterine isthmus, and an absence of healthy myometrium between the bladder and the sac.14 Some authors suggested an additional criterion of prominent peri-trophoblastic flow in the diagnosis of CSP.15 However, misdiagnoses still occur. Lai16 reported difficulty in making a differential diagnosis between an ectopic pregnancy implanted in the oviduct or in the myometrium of a previous CSP, even as early as the 7th week of gestation. They suggested operative laparoscopy as a diagnostic tool. On the other hand, Godin et al reported the use of MRI to confirm a diagnosis of CSP.17 MRI or three dimensional-colors Doppler imaging can enhance the diagnostic accuracy by evaluating the flow, resistance and pulsatility indices in the peri-trophoblastic vasculature; however, images may still resemble placenta accreta later in pregnancy.6 Because of the rarity of the condition, the majority of CSPs are case reports or small case series reported in the literature, with no consensus on the preferred mode of treatment. Generally, termination of pregnancy in the first trimester is strongly recommended to avoid life-threatening complications.18 Treatment objectives should be to perform feticide prior to rupture, to remove the gestation sac and to retain the patient’s future fertility. MTX has been used extensively as first-line treatment in cases of tubal and cervical pregnancy if gestational age is less than 9 weeks, fetal pole size does not exceed 10 mm, no embryonic cardiac activity is seen, and serum β-hCG levels are less than 10,000 IU/L.19 Experience regarding the efficacy and dosage is limited in CSP. A single dose of systemic MTX has been used to treat CSP.20,21 However, others have claimed that systemic multi-doses of MTX are necessary for successful management.22,23 Such treatment was initially employed for the management of CSP as an adjunct to hysterotomy or other procedures. Later, however, it was used as a primary therapy, for cases diagnosed early. Slow drug absorption into the CSP after systemic methotrexate is expected because the pregnancy is surrounded by fibrous scar rather than a normally vascularized myometrium, potentially limiting systemic access. In our study, all patients responded well to systemic MTX. Rotas et al.,8 reported 16 cases that were treated only by systemic intramuscular methotrexate; in 5 [36%], all with baseline β-hCG level less than 5,000 mIU/mL, it led to a complete and uncomplicated resolution within a few months. Another 5 women received multiple doses of intramuscular MTX alternating with leucovorin. Treatment was successful in 3 of the women; in the remaining 2 women, it was complicated by hemorrhage that was managed by laparotomy and wedge resection in one and hysterectomy in the other. The mean resolution time for CSP in our study was 52 days [range 27-67 days]. Wu24 found the decrease of serum β-hCG levels in patients receiving intramuscular MTX injection was slow, and only four [16%] patients had a 50% drop in their β-hCG levels after the first dose of MTX. The study by Yang25 also found the time for β-hCG level to decline to normal was long in CSP patients receiving intramuscular MTX injection only. Since the decline of β-hCG level may very slow, and uncontrolled vaginal bleeding could take place or continue at any time, and it was hard to predict when the CSP mass completely resolved if treated with MTX only. Only one treatment course of MTX was needed in 7 of our patients [58%], while two courses were needed in the remaining 5 [42%]. Using serum β-hCG level as an indicator for the need for further treatment, Chen26 found a 68.3% decline of β-hCG level in one case, but only a decline of 49.6% in the other case, 1 week after treatment, levels of β-hCG were high [33,082 mIU/mL] after treatment in the later case, prompting two additional courses of systemic MTX, while the first case received only one course of systemic MTX therapy. These results suggested that the number of treatment courses required might be related to gestational age at diagnosis and to the presence of a fetal heartbeat. Before MTX treatment, the mean of serum β-hCG levels of our patients was 13765.8mIU/mL. Unlike the experience with single-dose MTX treatment in tubal pregnancies that indicate a higher failure rate for patients with an initial β-hCG level greater than 10,000mIU/mL. Fertility outcome after treatment of ectopic pregnancy is one of the key factors when choosing treatment strategy. We did not asses the overall success rate or the outcome of subsequent pregnancies after treatment for CSP. Several authors have reported uneventful subsequent pregnancy course after surgical repair,27 but the integrity of the uterus after medical treatment is still unclear. Finally, although the medical treatment of CSP of our 12 patients was successful, the evaluation of treatment results based on the outcome of only 12 cases is not sufficient to draw a final conclusion regarding the efficacy of such treatment modality, especially also because there was no direct comparison with other available treatment options. So in view of the increasing rate of CS, health care providers should be aware of the possibility of this ectopic pregnancy type in subsequent pregnancies.
None.
The author declares there are no conflicts of interest.

©2019 Altraigey, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.