MOJ
eISSN: 2475-5494


Review Article Volume 12 Issue 2
1Obstt & Gynae, specialist reproductive endocrinology & Infertility specialist, Scientific Director cum Owner Dr Kulvinder Kaur Centre for Human Reproduction 721, G.T.B. Nagar, Jalandhar-144001, Punjab, India
2Obstt & Gynae, specialist reproductive endocrinology& Infertility specialist, Ex-Rotunda-A Centre for Human Reproduction 672, Kalpak Garden, Perry Cross Road, Near Otter’s Club, Bandra(W)-400040 Mumbai, India
3Consultant Neurologist, Swami Satyanand Hospital Near Nawi Kachehri, Baradri, Ladowali road, Jalandhar Punjab, India
Correspondence:
Received: June 23, 2023 | Published: August 2, 2023
Citation: Kaur KK, Allahbadia GN, Singh M. Brown adipose tissue in the form of innovative approach for polycystic ovary syndrome treatment-still long time to reach clinical arena: a narrative review. MOJ Women’s Health. 2023;12(2):31-39. DOI: 10.15406/mojwh.2023.12.00316
Brown adipose tissue(BAT)portrays a specialized tissue, possessing a crucial part in metabolism as well as energy expenditure(EE) via adaptive non shivering thermogenesis .Recently it has assumed a significant part in the treatment of obesity along with metabolic disease.The thermogenesis action of BAT is brought about by uncoupling protein1 (UCP1 ),that uncouples adenosine triphosphate (ATP) generation from oxidation of energy substrates.Having reviewed earlier various aspects of Polycystic ovary syndrome (PCOS) pathophysiology,treatment,role in trans generational PCOS transferalong with role of BAT pathophysiology,beige/brite adipocytes in the treatment of obesity along with metabolic disease here we decided to further evaluate the possible part of (BAT in PCOS. Thus anarrative review was carried out using the pubmed, Web of Science , Medline, Embase, Cochrane reviews, and Google Scholar, Search engine with the MeSH Terms;PCOS; impaired lipid metabolism; Brown Adipose tissue (BAT); White Adipose tissue(WAT); oxidative stress;inflammation;obesity ;T2DM); Type 1 diabetes (T1D); role of natural substances for PCOStherapy like rutin ,berberine;resveratrol ; weight reduction; browning of WAT ;Macrophage Polarization from 1990 till date in 2023.We found a total of 250 articles ,out of which we selected 100 articles for this review.No meta-analysis was done.The endocrine action of brown Adipocytes impacts the energy balanceof glucose as well as lipid homeostasis thus impacting the correlation of BAT activity along with metabolic profile . PCOS mirrors a , complicated reproductive as well asmetabolic condition of women in their reproductive age . functional aberrations in adipose tissue have been illustrated in PCOS patients .Multiple studies have illustrated that BAT possesses the capacity of controlling the properties of PCOS as well as escalating BAT mass/activity were efficacious in the therapy of PCOS. Via cold stimulation,BAT transplantation as well as activationwith substances like rutin hypoglycemia can be attained .
Keywords: polycystic ovary syndrome (PCOS), brown adipose tissue (BAT), bAT transplantation, rutintreatment, cold exposure
Adipose tissue (AT) which is inclusive of White Adipose tissue (WAT), brown adipose tissue (BAT), beige adipose tissues (Be AT) carry out necessary functions regarding sustenance of whole –body energy homeostasis.1 BAT reflects a fat tissue that has become specialized regarding adaptive non shivering thermogenesis for heat production on exposure to cold stress. BAT takes part in primary metabolism as well as energy expenditure (EE) along with can be promptly stimulated with utilization of thermal in addition to dietary stimuli.2 As per a recent study it was illustrated that an escalation in body mass along with function might work in the form of an eficacious therapeutic target in the context of obesity in addition to other associated metabolic diseases in such cases.3 BAT depots got accidentally discovered in inter scapular areas of smaller mammals and human infants by the utilization of 2‒deoxo‒2‒ Fluoro‒D–Glucose (18F‒FDG)/ positron emission tomography (PET)/ computed tomography(CT) scanning methods.4–7
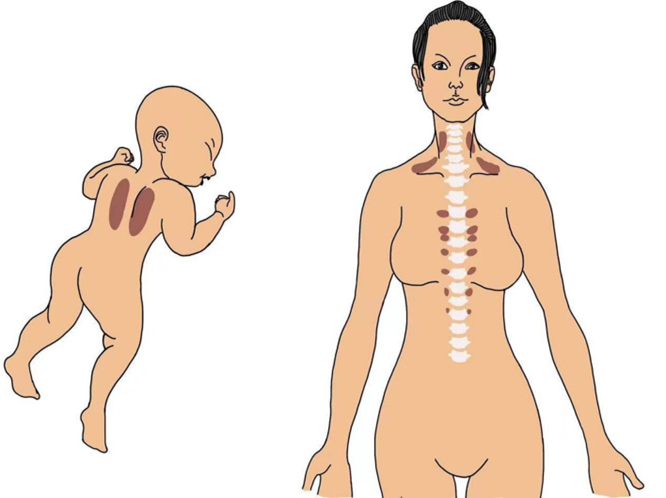
Figure 1 Courtesy ref no7 A schematic representation of BAT occurrence in the interscapular region in an infant and in the supraclavicular, cervical, and paravertebral regions in an adult human.
Detection of BAT was further feasible by these scanning methods in particular in supraclavicular,cervical and paravertebral areas of adults in neck area (biopsy proven).4,5 These scanning methods of PET -CT illustrated a robust positive association amongst BAT action along with basal metabolic rate(BMR). Additionally, BAT activation possessed an inverse association with age, body mass index (BMI)8 as well as adiposity in adults. The young along with lean females have greater metabolically active BAT.9 Brown adipocytes possess individual characteristics, like multiloculated small lipid droplets, with upregulated uncoupling protein1 (UCP1), enrichment of mitochondria, capillaries.3 BAT thermogenesis is basically based on UCP1, a (biomarker for BAT) on the mitochondrial inner membrane for energy dispersal.10,11 UCP1 results in uncoupling of adenosine triphosphate (ATP) generation from oxidation of energy substrates, thereby facilitating non generational EE via escalated mitochondrial uncoupling.5,9,10 The action as well as formation of brown adipocytes are controlled by the sympathetic nervous system(SNS) thermogenesis is regulated by the leptin melanocortin pathway.12,13
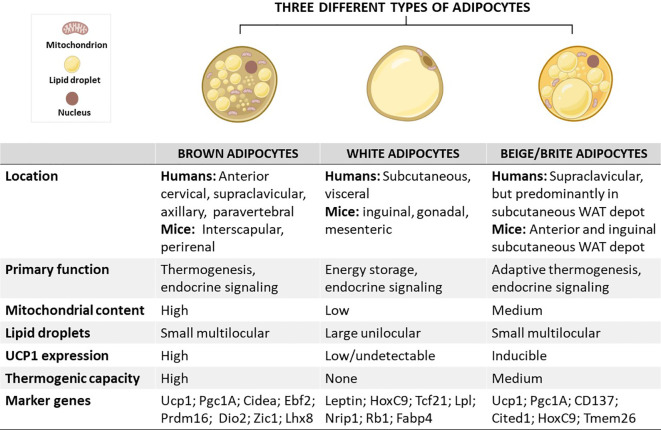
Figure 2 Courtesy ref no-14 Schematic overview showing the characteristics of brown, white, and beige/brite adipocytes, including their localization, morphology, physiological function, and marker genes.
In brief, the anatomical location of these adipocytes differs, except for white and beige adipocytes which are found within WAT depots. In terms of morphology and function, brown adipocytes are comprised of small multilocular lipid droplets, high mitochondrial content, and increased UCP1 expression, which can be also induced in beige adipocytes to promote thermogenesis and enhance energy expenditure, features that are important for the treatment of obesity. In sharp contrast, white adipocytes have fewer mitochondria and large unilocular lipid droplets to facilitate the storage of energy in a form of fats. Of note, these adipocytes have similar and distinct marker genes which could play an important role in tracking metabolic complications linked with obesity. Cidea, cell death-inducing DNA fragmentation factor-like effector A; CD137, tumour necrosis factor receptor superfamily, member 9; Dio2, Iodothyronine deiodinase 2; Lhx8, LIM homeobox protein 8; Pgc1A, peroxisome proliferator-activated receptor coactivator 1 alpha; Prdm16, PR domain-containing 16; Tcf21, Transcription factor 21; TMEM26, transmembrane protein 26; UCP1, uncoupling protein 1; WAT, white adipose tissue; Rb1, RB transcriptional corepressor 1; Zic1, zinc finger protein of the cerebellum.
Earlier we had reviewed regarding different classifications and treatment strategies in addition to role of Antimullerian Hormone as an early predictor of PCOS in perimenarchal girls, part of AMH in dysfunctional folliculogenesis in addition to impairment of gonadotropin control, apart from its implication in transgenerational transfer of PCOS, role of flutamide in identification of women of Polycystic ovary syndrome(PCOS) at risk for generation of Metabolic disease in normal weight PCOS ,role of kisspeptins regarding answering the modes by which transgenerational transmission of Polycystic ovary syndrome(PCOS) occursto find a way of avoidance of inheritance of PCOS.15–28 Here we considered how this knowledge might be applied of browning of WAT in treating PCOS.
Thus anarrative review was carried out using the pubmed, Web of Science , Medline, Embase, Cochrane reviews, and Google Scholar, Search engine with the MeSH Terms;PCOS; impaired lipid metabolism; Brown Adipose tissue (BAT); White Adipose tissue(WAT); oxidative stress; inflammation; obesity ;T2DM); Type 1 diabetes (T1D);SGLT2 inhibitors ; role of natural substances for PCOS therapy like rutin ,berberine; resveratrol ; weight reduction; browning of WAT ;Macrophage Polarization from 1990 till date in 2023.
We found a total of 250 articles, out of which we selected 100 articles for this review. No meta-analysis was done.
BAT liberation factor - adipokines cause improvement of metabolic health
Brown adipokines
Brown adipokines are controlling factors liberated by brown adipocytes possessing autocrine, endocrine as well as paracrine actions along with control BAT differentiation.12 Certain adipokines reveal hormonal actions that escalate BAT actions, result in improvement of metabolic characteristics of glucose as well as lipid homeostasis apart from modulation of browning of WAT.13 Furthermore, thermogenic stimuli, stimulate brown adipocytes to liberate signalling molecule that target the sympathetic nerves, vasculature, immune cells regarding tissue remodelling. Adipokines further stimulate organs in addition to cells located distally for performance of different local along with systemic functions.12,29

Figure 3 Courtesy ref no-7 A Adipokines secreted from BAT. Contribute to the regulation of various functions. FGF-21, fibroblast browth factor 21; BMP-8b, bone morphogenetic protein 8b; IL-6, interlukin-6; NGF, nerve growth factor; NRG-4, neuregulin 4; IGFBP2, insulin-like growth factor-binding protein 2; VEGF-A, vascular endothelialgrowth factor A; IGF-1, insulin growth factor-1, METRNL, meteorin-Like; CXCL14, chemokine (C-X-C motif) ligand 14; GDF-15, Growth and differentiation factor 15; SLIT2-C, C-terminal fragment of SLIT-2.
Moreover, BAT possess the capacity of liberating signaling peptides,lipokines along with exosomal microRNAs for controlling metabolism in organs located distally regarding coordinating metabolic working of whole body.30,31 BAT aids in metabolic homeostasis through UCP1 modulated thermogenesis along with liberated cytokines like Fibroblast growth factor 21(FGF21) in addition to adiponectin.32 One hundred and one full proteins were observed from the secretome of brown adipocytes along with ependymal -related protein 1(EPDR1) which act in the generation of brown adipocytes33 BAT thermogenesis might significantly influence the long term control of energy homeostasis apart from body weight; thereby an escalation of BAT volume as well as/or action might become an innovative therapeutic strategy for the treatment of patients with obesity in addition to metabolic diseases.
Therapeutic capacity of BAT
BAT portrays a robust sink in the context of elimination along with oxidation of glucose and triglycerides(TG) from blood, thereby it possesses therapeutic probability of treatment of variable metabolic diseases via its antiglycemic, antilipidemic in addition to antiobesity action.4,34 Brown fat possesses the capacity of generating 300 time greater heat/unit mass in contrast to any different organ in the body subsequent to maximum stimulation, that is implicated in about 10% of the heat generated/day.35 Of the maximum stress which is laid regarding BAT associated pharmacologic research is obesity treatment which takes place secondary to continued energy imbalance in view of escalated calorie ingestion that far exceeds the calorie expenditure, as a sequence resulting in different variety of metabolic diseases inclusive of hyperglycemia, Type2 Diabetes mellitus(T2D) along with hyperlipidemia.36 Vascular lipoprotein homeostasis is controlled by BAT through escalating triglyceride rich lipoprotein(TRL)turnover in addition to transportation of lipids into BAT.37 Furthermore, BAT possesses therapeutic capacity of treatment of cardiovascular disease (CVD) in view of its actions of escalating fatty acid catabolism in addition to decreasing triglycerides, atherosclerosis along with inflammation in the case of obese patients.38 Additionally, stimulation of BAT, beige adipocytes potentially liberate insulin like growth factor binding protein (IGFBP1), that possesses anabolic action on bone tissue, thereby it might possess effectiveness in the treatment of skeletal abnormalities.38
Considering in toto human thermogenic adipocytes might work in the form of therapeutic targets via 3 separate strategies i) an escalation in BAT mass by induction of progenitors ii) an escalation in WAT browning by escalating the generation of beige adipocytes iii) through enhancement of BAT working via upregulation of its controlling pathways.39
WAT browning
BAT in addition to BAT mirror the primary regions in the context of the adaptive non shivering thermogenesis as well as are key for metabolic control via liberation of adipokines in reaction to various pathophysiological stimuli. Canonical brown adipocytes have their placement in generationally programmed depots in rodents as well as human infants. Beige(coined from ‘’brown in white’’) adipocytes portray brown adipocytes, which in reaction to thermogenic stimuli like chronic cold exposure, go through browning event in white fat that are significant constituents of BAT depots in adults along with might become an innovative therapeutic target in the context of therapy of obesity ,insulin resistance(IR) in addition to Type2 Diabetes mellitus(T2D).40 No proof was illustrated that the thermogenic working along with modes are variable amongst beige from brown adipocyte(see Figure2).41 WAT possesses single lipid droplet, lesser mitochondria, besides has no UCP1.42 WAT is implicated in fat storage in the form of energy. Energy storage is as triglycerides and is responsible for the generation of obesity along with numerous metabolic diseases.43 Furthermore, WAT liberates energy in body as free fatty acids (FFA) as well as glycerol.44 WAT inclusive of subcutaneous fat along with visceral fat can go through browning. Nevertheless, the subcutaneous fa t(like inguinal) possess in particular, proneness to browning as well as illustrated significant escalated UCP1 quantities .The browning of WAT in the form of therapeutic approach usually points to long term treatment with Peroxisome Proliferator Activated Receptor (PPAR)agonists as well as escalates the generation of beige adipocytes.40
BAT activation
The SNS regulates BAT activity along with BAT activation takes place through metabolic as well as hormonal signals.11,45 EE takes place on activation of brown along with beige fats in addition to diminishes hyperglycemia as well as hyperlipidemia. BAT in addition to BAT activation result in escalated lipolysis along with hampers the event of autophagy along with mitophagy.46
Exposure to cold
Continued / chronic cold exposure might enrol along with result in BAT activation with escalated EE as well as lead to pacey glucose along with lipids oxidation. As per certain studies exposure to cold leads to induction of UCP1, with this action being greater in women in contrast to men.47 BAT activation takes place in extrauterine milieu along with robust endocrine stimulation at the time of birth.2,48 Conversely, acute along with recurrent exposure to mild cold (about17-190C) might escalate the volume in addition to BAT activity in adults. These actions are modulated by transient receptor potential(TRP)channels.
Diet Induced thermogenesis (DIT)
Apar from cold exposure consumption of meals, in particular that has enrichment of protein besides chemicals like capsaicin might lead to induction of BAT thermogenesis alias DIT along with portrays comparatively big constituents of full days EE.49 Members of the TRP usually work in the form of chemical receptors of plant apart from food metabolites like TRP vanilloid1( TRPV1) agonists inclusive of capsaicin as well as capsinoids which might simulate the actions of cold exposure on diminished body fat through activation and enrolment of BAT. The anti-obesity action of food ingredients inclusive of catechins present in tea might take place via the TRP- SNS- BATaxis activation.50
More channels
Other than DIT, BAT activity might be escalated through central in addition to peripheral effects. Like thyroid hormone possesses the capacity of activating BAT centrally via binding to thyroid receptors present in brown adipocytes that directly lead to induction of expression of thermogenic genes. The neurotransmitter orexin possesses the capacity of escalating BAT working by modulation of sympathetic outflow in addition to induction of the differentiation of brown fat precursors.51 β3-adrenergic substances have the capacity of activating BAT thermogenesis apart from inducing WAT browning 52 Thiazolidenediones, a PPARγ activator might stimulate WAT browning by enrolment of already present BAT depots,53,54 however their actions are based on simultaneous activation of noradrenergic signals by efficacious thermogenic induction.55 Certain studies have illustrated that irisin along with melatonin possesses the capacity of activating BAT as well as transplanting brown adipocytes stem cells;which might escalate thermogenesis from brown and beige adipocytes.56 Ginseng extract(GE) has the capacity of activating BAT as well as escalating metabolism.57 Metformin might result in improvement of UCP1 quantities along with causing mitochondrial biogeneration in the BAT; nevertheless it is not efficacious regarding body mass.58 For the differentiation of brown adipocytes for numerous receptors/ transcription factors inclusive of Peroxisome Proliferator Activated Receptor γ( PPARγ), besides Peroxisome Proliferator Activated Receptor γ coactivator -1α( PGC-1 α ), PR (PRD1‒BF‒RIZ1 homologous) domain containing 16(PRDM16), CCAAT/enhancer binding protein (C/EBP β ), Bone morphogenetic protein7 (BMP7)for promoting acquiring of thermogenic phenotype of BAT that gets finally modulated by UCP1.6 BAT further might be activated by natriuretic peptides FGF21 as well as BMP 8b.51
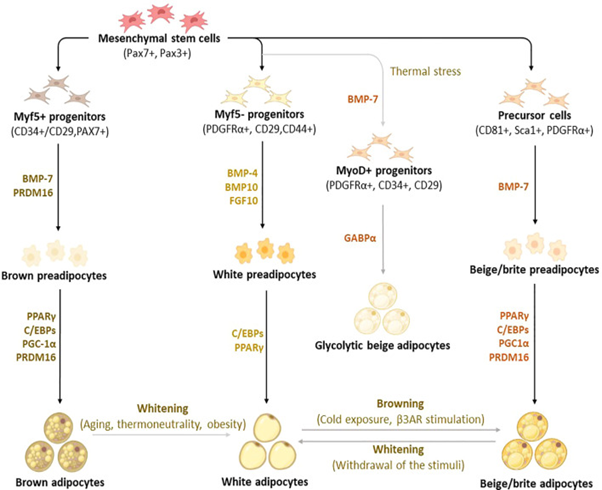
Figure 4 Courtesy ref no-14 Differentiation and trans differentiation trajectories of major adipocyte populations, including brown and white adipocytes, as well as canonical and glycolytic beige/brite adipocytes. Generally, brown adipocytes are derived from Myf5+ progenitors, whereas white and beige adipocytes originate from Myf5- progenitors that descend from mesenchymal stem cells.
New advances on adipocyte origin show that there is another subpopulation of beige adipocytes known as glycolytic beige adipocytes, which are derived from MyoD+ progenitors within the stromal vascular fraction of inguinal WAT in response to thermal stress. During differentiation secretory factors BMP-7 and transcriptional factor PRDM16 induce Myf5+ cell commitment to brown preadipocytes whereas BMP-4, BMP-10, and FGF10 induce Myf5- cell commitment to white preadipocytes. On the other hand, BMP-7 induces a commitment of beige adipocyte precursors to canonical beige preadipocytes. Differentiation of all types of preadipocytes into mature adipocytes is driven by PPARγ, and C/EBPs, whereas brown and beige adipocytes require the expression of additional factors, including PRDM16 and PGC1α. Moreover, GABPα regulates the differentiation of glycolytic beige adipocytes. During transdifferentiation, white adipocytes convert to beige adipocytes following cold exposure or β3-AR stimulation. Likewise, brown and beige adipocytes can transdifferentiate into white-like adipocytes, a process termed whitening. β3-AR, beta-3 adrenergic receptor; BMP, bone morphogenetic protein; C/EBPs, CCAAT/enhancer-binding proteins; GABPα, GA-binding protein alpha; PGC1α, peroxisome proliferator-activated receptor coactivator 1 alpha; PPARγ, peroxisome proliferator-activated receptor-gamma; PRDM16, PR domain-containing 16; UCP1, Uncoupling protein 1infertility.
BAT in the form of innovative therapeutic approach for Polycystic ovary syndrome (PCOS) treatment
PCOS
PCOS mirrors a medical disorder correlated with abnormalities in metabolic, reproductive as well as psychological functions,impacting about 20-25% of women of reproductive age.59,60 Its manifestations are in the form of a heterogenous problem implicating menstrual cycles aberrations,anovulation, insulin resistance(IR) hirsutism, androgenic alopecia.61 Hyperinsulinemia as well as IR possess a significant part regarding its pathophysiology in addition to metabolic aberrations of PCOS.62 Epidemiological studies have illustrated that 38-88% of PCOS women possess central adiposity, overweight or obesity.63 PCOS women with hyperandrogenism along with IR have the prediliction for visceral fat hypertrophy apart from early initiation of dysfunctional glucose tolerance test exists in 30-40% ,T2D in 10%, borderline or escalated lipid quantities in 70% in addition to metabolic syndrome(MetS) in 50%.64 Thereby PCOS is intricately associated with metabolic abnormalities along with is believed to be a metabolic condition.65 Moreover, PCOS is correlated with dysfunctional AT physiology as well as possesses greater risk of non alcoholic fatty liver disease( NAFLD) generation.66,67 Furthermore PCOS women have proneness for generating endothelial impairment, CVD, atherosclerosis with a 2.7 times escalated risk of endometrial carcinoma production.68 Additionally, PCOS might escalate the risk of formation of anxiety, depression as well as sleep disturbances that diminishes quality of life (QOL), in particular in patients that have hirsutism, weight accrual or hirsutism acne.69 PCOS women possessing infertility have the maximal inimical QOLin view of psychological along with emotional stress.70
PCOS management in general
PCOS possesses important clinical presentations inclusive of different kinds of psychological , reproductive and metabolic characteristics.71 The newer International Evidence Based Guidelines for the assessment and management of polycystic ovary syndrome emphasized on the significance of lifestyle management inclusive of diet ,exercise along with behaviour in the form of initial line of management regarding improvement of signs as well as symptoms of PCOS.72 Just 5% of body weight reduction is enough to illustrate improvement of insulin sensitivity, hyperandrogenism, menstrual aberrations as well as other metabolic along with reproductive characteristics clinically.73
Additionally, for hyperandrogenism management, as well as /or menstrual irregularities in case of PCOS it is advocated combined oral contraceptive (COC) pill be used.54 Metformin combined with lifestyle alterations management might possess the capacity of improvement of weight, hormonal as well as metabolic results along with might be associated with greater advantages in patients at risk for diabetes as well as dysfunctional glucose tolerance.74 Apart from lifestyle alterations, anti obesity agents might be utilized for obese patients with PCOS.59 Thereby metformin is advocated as a lone agent or combined with inositols basically for metabolic disorders. At present inositol which is an experimental treatment might be taken into account for PCOS treatment.75 Statins are believed to be safe regarding dyslipidemia treatment in patients with PCOS.76 Regarding treatment of anovulation as a cause for infertility, aromatase hampering agents like letrozole are advocated in the form of initial line of treatment, with Clomiphene Citrate as well as lone metformin or in combination.77,78 Gonadotropins are considered as 2nd line of treatment.79
BAT in the form of PCOS treatment
It has been illustrated in studies that there is a reduction in BAT activity in patients with PCOS along with in a rat model of PCOS with the probability of escalated adiposity80 as well as major presentations of IR along with inflammation.81 Impairment of AT facilitates metabolic situations in the peripheral tissue of PCOS patients possessing adipocytes of greater size, lesser activity of lipoprotein lipolytic enzymes in addition to dysfunctional capacity of catecholamine modulation of lipolysis.82 Moreover, diminished EE might be associated with reduced BAT function in female mice with PCOS.83 Thereby the probability of BAT activation in the form of a therapeutic approach has to be taken into account for treatment of PCOS for the reversal of metabolic situations.73,84
Cold exposure of BAT might escalate EE along with result in whole –body energy reduction.84 Frequently cold stimulated BAT activity is observed in the case of humans, with a prevalence of 30-100% based on cohort studies.85 Escalation of BAT activity is feasible by reduction of the atmospheric temperature or by planning cold exposure in areas where humans reside that might further diminish body fat.86 Nevertheless, the advantages of cold exposure were not constantly observed in humans as well as of animals87 A recent study illustrated that exposure to cold uptill 40Cfor 20 days resulted in reversal of the acyclicity of the oestrus cycle along with diminishing the quantities of testosterone as well as Luteinizing hormone(LH) by activation of endogenous BAT in case of rats having PCOS.88 Moreover, the expression of steroidogenic enzymes as well as theinflammatory factors were diminished significantly in the ovaries of rats having PCOS. Histological evaluation illustrated that cold exposure resulted in significant improvement of infertility along with ovulation, diminished cystic ovarian follicles generation along with escalation in the corpus luteum in case of rats having PCOS.89 These observations pointed that cold exposure further might be an innovative approach for PCOS therapy.
Brown adipose tissue transplantation possesses the capacity of normalizing glucose tolerance in addition to diminishing tissue inflammation along with markers of T2D like polydipsia, polyuria as well as polyphagia, resulting in euglycemia. These actions are insulin independent however their actions are associated with BAT recovering in mice.90 BAT transplantation was efficacious in improvement of energy metabolism, as well as insulin sensitivity, avoidance of weight accrual stimulated by high fat diet(HFD), along with reversal in prior presence of obesity in mice.89 Significant improvement took place in IR as well as liver steatosis subsequent to BAT transplantation along with diminished body weight with escalated oxygen utilization in addition to reduction of full body mass in ob/ob mice.91
Recovering of BAT activity might result in improvement of PCOS with numerous studies have illustrated that BAT transplantation caused reversal of PCOS.92,93 BAT transplantation might further significantly escalate endogenous BAT activity along with enhance the quantities of circulating adiponectin as well as insulin sensitivity, thus ultimately abrogating hyperandrogenism, acyclicity of polycystic ovaries along with infertility in case of rats having PCOS.92 Additionally, BAT transplantation, rescued PCOS phenotypes in a dramatic fashion that is in parallel with documented outcomes of adiponectin protein delivery.93 A recent study illustrated that BAT xenotransplantation in rat might significantly aid in recovery of ovarian function along with fertility in PCOS mice.92
Clinically BAT activation by long time cold exposure as well as BAT transplantation apparently does not work in maximum patients of PCOS. Thereby endogenous BAT activation with utilization of natural substances might be efficacious strategy regarding the treatment of PCOS. Treatment with rutin (akin to a citrus flavonoid glycoside observed in buck wheat) for 3 wks have further been documented to escalate BAT activation,which leads to improvement of thermogenesis, as well as insulin sensitivity in case of rats having PCOS.94 Furthermore, the expression of steroidogenic enzymes were upregulated inclusive of steroid 17-αhydroxylase /17,20 lyase(P450C17), aromatase, 3 βhydroxysteroid dehydrogenase(3HSD) alongwith17βhydroxysteroid dehydrogenase1(17HSD), steroidogenic acute regulatory protein (STAR). Additionally, rutin treatment normalized acyclicity in addition to quantities of serum LH as well as a big quantity of mature ovulated follicles were seen with diminished cyst generation in rats with PCOS.94 Another study illustrated that rutin might possesses the capacity of escalating BAT activity as well as induction of beige adipocytes, therefore mitigating obesity along with IR in case of obese mice.95 Plenty of examination has been done to discover substances that possess the efficacy of activating BAT in the context of PCOS treatment.
Further assessment of role of exercise
Basic role of exercise-role of irisin, exercise on neurogenesis and BDNF
Wrann et al investigated the effects of exercise on fibronection type III domain containing 5 (FNDC5) expression and function using an established endurance exercise regimen;namely 30days of voluntary free running wheel exercise. This regimen is known to induce BDNF expression, neurogenic, dendritic spines and improved memory function in mice.24 The training was sufficient to induce muscle fndc5 gene expression as well as transcriptional regulators pgc-1α, Err –α, known mediators of the exercise response in skeletal muscle. They showed that FNDC5 is elevated in endurance exercise in the hippocampus of mice and that PGC-1α and FNDC5 regulate BDNF expression in the brain.24
Probable part of exercise and apelin in pregnancy complications avoidance
The prevalence of maternal obesity at the time of pregnancy is correlated with the risk of generation of gestational diabetes, preeclampsia, in addition to cardiomyopathy. Environmental factors like active lifestyles along with apelin might result in advantages alterations. Earlier we had reviewed the role of apelin. Recently apelin an adipocyte derived hormone, by apelin-AP1 signaling was reported to promote brown adipocyte differentiation, bothby increasing brown adipogenic as well as thermogenic transcriptional factors via the PI3K/Akt and AMPK signalling pathway by Than et al. Apelin, a myokine gets formed by fat tissue as well as muscle along with in obesity its reduction takes place. Moreover, its expression takes place incentral nervous system (CNS)[ hypothalamus], heart as well as stomach. It possesses significant anti-inflammatory part, aiding in the regulation of heart muscle along with in turn blood pressure(BP), besides cell cycle control along with demise. This organokine further stimulates muscle regeneration, with avoidance of muscle depletion.24,96
Recently Pehlwani97 studied the part of rats apelin as well as exercise (45 to 65% VO2max for 6 to 9 weeks) in rats at the time of pregnancy which escalate brown adipose tissue (BAT) proteins like cell death inducing DNA fragmentation factor (Cidea), elongation of very long chain fatty acids like 13 (Elovl3), UCP1, PRDM16, and PGC-1a in males and females foetuses, whereas white adipose tissue (WAT) is diminished. In humans as well as animals apelin along with exercise stimulate the expression of the glucose transporters (GLUT1/2/4) in the muscle in addition to AT via the PI3K/Akt along with AMPK pathways. Hence, exercise along with apelin might be acknowledged as controllers of energy metabolism as well as possessing anti-obesity as well as antidiabetic characteristics. In mice, exercise further generates a short-term hypoxic milieu in the pregnant mother; activating HIF-1, VEGF, and VEGFR, as well as escalating angiogenesis. Exercise and apelin further escalated vasodilation, angiogenesis, along with repression of inflammation via the L-arginine/eNOS/NO pathway in humans. Exercise can stimulate the ACE2-Ang-(1-7)-Mas axis in parallel with hampering the ACE-Ang II-AT1 pathway. Exercise as well as apelin apparently avoid generation of preeclampsia via these events. In rats, moderate-intensity exercise (60 to 70% VO2max for 8 weeks) and apelin/APJ further might avoid pathological hypertrophy in pregnancy by activating the PI3K/Akt/mTOR/p70S6K pathway, PI3k-Akt-ERK1/2-p70S6K pathway, in addition to the anti-inflammatory cytokine IL-10. In view of pre-clinical studies have been attempted greater in animal models, future research with scientific guidelines need to dedicate greater attention to humans. In future research, time factors such as the first, second, and third trimesters of pregnancy as well as the extent in addition to time period of exercise are significant variables that need to be taken into account for estimating the ideal intensity as well as and of exercise.
Part of resistance training
In a study by Kite et al,98 where they evaluated part of resistance training in women who could not tolerate aerobic exercises in contrast to healthier ones who could perform them .They concluded that statistical alterations were documented to be associated with metabolic(like glycemia along with fat free mass ) as well as hormonal (like T, in addition to sex hormone binding globulin(SHBG),profiles .As per their conclusions absence of enough studies regarding this arena, they illustrated that the degree of alterations was minimal and although documented statistically significant for numerous results ,the revealed results of alterations along with quality of proof is queried. Thus stressed on how good fashioned / documented as well as adequately powered trials are required.99
Further evaluated capability, opportunity, and motivation (COM), Identifying constructs for increasing physical activity (PA) behaviours in PCOS women. Of the 333 patients having enrolment, who fitted eligibility criteria documented advantageous alterations of BMI, depression, mental happiness, health that was self realized ,robustness of insomnia. COM scores escalated per PA categories, whereas ordinal logistic regression (OLR) isolated conscious along with automatic motivation which reasoned out the greater PA variability. The maximum active patients who took part documented advantageous results. Nevertheless, estimation of greater conferred protection or ill health works in the form of a hurdle was not feasible. Thereby these observations pointed that further behavioural intervening is warranted regarding escalated patient motivation.99
Diet physical activity (PA) lifestyle behaviours, nutrients
Kazemi et al100 aimed to assess the dietary along with physical activity behaviours in women with and without PCOS. Primary outcomes were inclusive of total diet quality, full energy consumption, along with full physical activity (PA), while secondary outcomes were inclusive of macronutrients, micronutrients, food groups, food glycemic indices, time spent sedentarily, as well as sitting. Basically their objective was to find if individual lifestyle behaviours could predict any predilection of weight accrual along with obesity in PCOS as well as targeted regarding exact nutrition along with PA intervening. The utilization of these observations might be used for future utilization regarding further advocates as well as conduct work to efficaciously tackle complications (weight gain, obesity, diabetes, infertility, CVD along with mental health) in this high-risk population.
A total of 333 participants were eligible; favourable differences were reported for body mass index, depression, mental wellbeing, self-rated health, illness perception, and insomnia severity for those reporting the highest PA levels. COM scores increased according to PA categorisation, whilst OLR identified conscious and automatic motivation as explaining the largest PA variance. The most active participants reported favourable data for most outcomes. However, determining whether health is protected by higher PA or ill health is a barrier to PA was not possible. These findings suggest that future behavioural interventions should be targeted at increasing patient motivation. Collective validation in in 39471total participants [ n=8736 PCOS;n=30735 controls) agreed that women with PCOS possess a lesser lower overall diet quality, inimical dietary ingestion (greater higher cholesterol, lesser magnesium and zinc) and lesser total PA, inspite of lesser alcohol ingestion in controls versus those without PCOS. Considerable heterogeneity among studies reinforces the need for research to address any relative contributions of other factors (e.g., genetic, metabolic or sociodemographic) to the variations seen. These clarifications might aid in future evidence-based guideline advocates on monitoring and managing PCOS in this era of precision lifestyle medicine.100
Thus, this review summarizes the generation along with propagation regarding the biology as well as pharmacological therapy for BAT regarding PCOS. We had already detailed that BAT possesses thermogenic probability for avoidance of obesity in addition to metabolic disease. Identification of major brown adipokines is highly significant in addition to their part for inventing innovative candidates along with efficacious therapeutic approaches for PCOS therapy. BAT possesses therapeutic probability of being a metabolic elixir in the form of antiglycemic, antilipidemic as well as whole body weight reduction. PCOS portrays a complicated reproductive in addition to metabolic endocrinology problem in women, being the major etiology of infertility with various presentations. Lifestyle management as well as behaviours, pharmacological therapies are of aid, however the efficacy is not parallel, not meeting patients requirements with PCOS. Multiple studies have illustrated that there is reduction of BAT activity in PCOS patients along with an enhancement of mass as well as/or BAT activity might prove to be efficacious in addition to provide innovative therapeutic strategies for PCOS treatment like cold stimulation, BAT transplantation in addition to activation with drugs . Nevertheless, the biggest limitation is most studies have been conducted in animals, with greater future work needed for unravelling the molecular mode preclinically for clinical validation. However maximum work is being pursued at present regarding physical activity, exercise in human PCOS women which indirectly activates BAT thus more stress has to be put on exercises, drug activation using rutin &further try other agents that brown like Berberine resveratrol, curcumin, flavonoids (quercetin)like rutin that has proven to be efficacious.96
None.
The authors declares that there is no conflict of interest.

©2023 Kaur, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.