MOJ
eISSN: 2641-9300


Review Article Volume 1 Issue 1
Kirschenweg 1, 86420 Diedorf, Germany
Correspondence: Vladimir F Niculescu, Cell biologist, Germany
Received: January 05, 2018 | Published: January 25, 2018
Citation: Niculescu VF. Carcinogenesis: recent insights in protist stem cell biology lead to a better understanding of atavistic mechanisms implied in cancer development. MOJ Tumor Res. 2018;1(1):18-29. DOI: 10.15406/mojtr.2018.01.00004
The present work substantiates the atavistic hypothesis of cancer and considers that cancer initiation starts from a mitotic- blocked precursor cell (protoprecursor) that escape amitotic death by a process of polyploidisation/depolyploidisation analogous to the encystment/excystment process of invasive pathogenic, amoebae. The protoprecursor cell down regulates its multicelular genes, up regulating unicellular gene networks of the dark genome; it primes a carcinogenic immortal self sufficient stem and progenitor cell lineage (caSPCL) developed and controlled by basic mechanisms of the common eukaryotic ancestor. The primitive cancer life cycle contains reproductive cyst-like structures (aCLS, PGCCs) protected by a more or less characteristic envelope. aCLS’s microcell progeny forms an atavistic stem cell line capable to convert in two antagonistic sublines: one is the neotic more hypoxic aCLS+ progenitor subline that differentiates multiple generations of reproductive aCLSs by asymmetric cell division, and the other is the more oxygenic vegetative/ somatic aCLS-/siCLS+ subline that do not form aCLSs in cultures however, may express differentiation potential in conditions of stress and genotoxic insults forming stress induced siCLSs respectively gCLSs. In primitive eukaryotes such as invasive, pathogenic amoebae, progenitor sublines for cycling encystment may transdifferentiate to further vegetative/ somatic sublines; vegetative sublines and clones may change the genotype. We redefine neosis as the atavistic reproductive life cycle of cancer that forms microcells (aCLS progeny) capable of stemness and caSPCL renewal. The aCLS+ subline is the neotic/carcinogenic subline: it generates the atavistic family of stem and progenitor cells (aCSCs).
Keywords: Cancer, Neosis, Atavistic stemness, Primitive cell lineages,Entamoeba, Genotoxic agents, DNA repair
aCSC, Atavistic Cancer Stem Cell; aCLS, Atavistic Cyst Like Structure; siCLS, Stress Induced aCLS; caSPCL, Carcinogenic Stem/Progenitor Cell Lineage; MGs, Multicelullar Genes; UGs, Unicellular Genes; DSBs, DNA Double Strand Breacks; DDR, DNA Damage Response Mechanism; nPGCC, Native Polyploidy Giant Cancer Cell; siPGCC, Stress Induced PGCC; gPGCC, Genotoxic Induced PGCC; ATD cyst, Autonomously Differentiated Cyst of E. invadens; ITD cyst, Stress Induced Cyst of E. invadens
As mentioned recently by Thomas et al.1 there are two main evolutionary hypotheses of cancer: the first theory proposes that cancer results from atavism and the second claims a somatic evolution by mutations (SMT). Evidence that confirms and expands the atavistic theory (AT) would be welcome because, alone, atavistic development could be successfully fought.
The somatic mutations theory
As shown by Soto and Sonnenschein2 the somatic mutation theory (SMT) considers that cancer is derived from a single somatic cell that has accumulated multiple DNA mutations. As a consequence of mutations in genes that control proliferation and cell cycle progression this somatic cell and its progeny switches from a state of quiescence-considered to be the default state of most cell proliferation in metazoa into a state of uncontrolled proliferation. The SMT has not been rigorously tested, and several lines of evidence raise questions that are not resolved by this theory. As more and more oncogenes and tumor suppressor genes have been identified in the course of time, further ad hoc postulates were added. One is the tissue organization field theory (TOFT) that considers that proliferation is the default state of all cells and carcinogenesis is a disease of tissue organization, comparable to organogenesis.3
The atavistic theory
The atavistic theory of cancer proposed by the physicians Davis and Line weaver in 20114 considers that the biological origin of cancer can be found in the early transitional phase from unicellularity to multi-cellularity, as early regulatory networks controlling the expression of unicellular (UG) and multi-cellular (MG) genes were instable. In 2012 this theory was strongly ridiculed by PZ Meyers (P-Zombie Meyers) in a rather unscientific manner (http://scienceblogs.com/pharyngula/2012/11/20/aaargh-physicists-again). According to the atavistic theory cancer is the release of highly conserved cell survival programs encrypted in the eukaryotic genome.1 It reflects the capacity of primitive single celled ancestors to respond effectively to stress and unfavorable conditions of life by reproductive encystment. Switching into the atavistic life form occurs by down regulation of MG genes while UG genes are strongly up regulated.5 Because of their common origin, modern day eukaryotes encript parts of the common ancestor genome. The “dark genome” of humans contains ca 104 to 2 x104 protein non-coding genes which have been inactivated in the course of evolution. It represents ca. 98.8% of cell’s DNA.6 Moreover, 1% of the human genome consists of “cancer genes” that are transcriptionally up- or down-regulated in cancer cells.7−10 These deep sleeping atavistic genes can be awakened by tumorigenic stressors. Trigos et al.5 investigate recently UGs and MGs expressed in tumors and found a close association between the age of the genes and their expression level.5,11 As cancer progresses from low to high grade, old genes are expressed at the highest level.11 In tumors, cancer cell populations are hierarchical and heterogeneous; Morii.12 differentiates sub-pools of cancer initiating cells (CICs) and non-CICs. CICs and non-CICs have stem cell characteristics possessing self-renewal and multi-differentiation potential. It is the same dual potential of self renewal and primitive terminal differentiation (PTD) conserved in the stem & progenitor cell lineage (SPCL) of invasive pathogen protists such as Entamoeba invadens.13 It is not quite clear how environmentally stressed human cells activate networks of silent atavistic dark genome genes mimicking complex protist life cycles. In any case, it seems that ancestral networks are still encoded in the human genome as highly dangerous atavistic/invasive programs.
The multi-hit hypothesis and the cancer platform model
In the past oncogenic “transformation” is thought to occur by accumulation of mutations and epigenetic events that activate oncogenes and deregulate tumor suppressor genes. Opponent oncogenes and proto-oncogens are implicated in the positive control of normal cellular growth while tumor suppressor genes are implicated in the negative regulation of the cellular growth. Mutations of tumor-suppressors inactivate gene function and are usually repressive 7,14however, they are insufficient to give rise to cancer. It cannot explain why the incidence of disease increases dramatically with age. The multiple-hit hypothesis was born as a consequence of statistical analysis. Analysts suggest that genes of the key regulatory pathways need four to five sequential “genetic lesions” in order to generate malignancy.15 Loss of differentiation and abnormal ploidy are cancer markers. Further markers are growth factors including membrane receptors (EGFR, PDGFR) and non-receptor proteins (ras or myc). The cancer platform model reduces cancer initiation to a mutual impact between increased cell proliferation and concomitant inhibition of cell death. The authors consider that proliferation and death could represent in fact an evolutionary response; deregulation of the proliferation - death balance may initiate cancer. Pedraza-Farina7 brought a third player in discussion namely the impaired terminal differentiation of cancer cells (tissular differentiation). Pedraza-Farina and other authors consider that oncogenes can promote terminal (tissular) differentiation mentioning that c-myc and ras are not already complementary.7,16−20 While several authors consider myc activation as a hallmark of cancer activation causing initiation of tumor and immortality and preventing metazoans terminal differentiation (Erenpreisa, personal communication) other authors consider myc activation as a consequence of oncogenic development and epigenetic events.21−23 Gabay et al.21 consider myc overexpression as “surprisingly incapable of inducing cellular proliferation or neoplastic transformation of most normal human cells”. myc activation alone does not induce tumorigenesis but has a destructive effect leading to mitotic cell cycle arrest, senescence and apoptosis. Although myc is part of the replication process that enforce replication and entry in the S phase it doesn’t favor cellular division. Cells undergo proliferative arrest and become polypoid. The consequences of myc activation depend on the physiological state of the cell, respectively the state of differentiation of particular cell lineages; myc cooperates with many other events in a permissive epigenetic context to initiate tumorigenesis by intrinsic mechanisms of proliferation and cell cycle control. The authors maintain that other genetic events and microenvironment changes may be required to perturb the regulation of cell inherent mechanisms.21
Carcinogenesis: a multistep/ multipath process
According to Siddiqui et al.24 neoplastic transformation preceding metastasis consists of separate but closely related stages: Cancer initiation involves the spontaneous alteration or change of genes induced by environmental and carcinogenic stimuli. Genetic and epigenetic changes result in deregulation of biochemical pathways associated with cellular proliferation and differentiation and a changed carcinogenic metabolism. Cancer promotion is considered to be a relatively lengthy process in which actively proliferating pre-neoplastic cells accumulate. Cancer progression is the transient phase between a pre-malignant lesion and the development of invasive cancer. It is the final stage of neoplastic transformation by genetic and phenotypic changes. In the course of their development abnormal, pre-cancerous cells gain new capabilities such as the ability to release growth factors and digestive enzymes promoting invasiveness. The effect of initiators is irreversible; it results in permanent genetic changes, also carried by the progeny. In the promotion phase altered cells and their progeny divide in a modified/uncontrolled manner leading to an excess (hyperplasia) of abnormal genetic/epigenetic modified cells. Cells and tissue do not look longer normal. (https://www.cancerquest.org/index.php/cancer-biology/cancer-development) Cancer researchers consider that tumors derive from a single cell clone while cancer cell populations consist of heterogeneous sub pools of cells with different features and functions.12
In recent years we have learned more about primitive stem and progenitor cell lineages (SPCLs) of protists. Protists such as Entamoeba and Giardia have primitive SPCLs hidden in their life cycle.13,25−29 Amoebiasis starts by infectious cysts excysting in intestine. Entamoeba’s mature innercyst cell is a tetranucleated 8N polyploid cell. It hatched out as a non-proliferative metacyst segregating its whole genome copies (WGC) to eight subnuclei that cellularize forming eight daughter cells (amoebulae, microcells). These microcells start a primitive multilined SPCL. Lineage’s cell lines and sublines proliferate by asymmetric cell division; symmetric division is rare. Entamoeba’s primitive SPCL consists of self-renewing cells, mitotic arrested/quiescent cells and reproductive cysts; the inner cyst cell is a primitive terminal differentiated (PTD) cell of reproductive function. Its progeny forms a transient undifferentiated stem cell line (former primary p-SRL) that converses to two more differentiated sublines: one is the reproductive progenitor subline (secondary s-SRL) producing autonomously ATD cysts by repetitive cyclic differentiation and the other a vegetative/invasive subline (tertiary t-SRL) not producing cysts. Apparently both sublines are antagonistic, however, the vegetative/invasive subline retains multiple differentiation potential. On the one side it is a clonogenic subline forming invasive clones with distinct genotypes (e.g. distinct hepatic genotypes); on the other side hypoosmotic stressors and nutrient free media induce vegetative/invasive cells to form ITD cysts. In the present work we use the term ATD+ subline for the s-SRL and ATD-/ ITD+ subline for the t-SRL subline of Entamoeba. Occasionally, the ATD+ subline transdifferentiates to a further ATD-/ITD+ subline. In low hypoxic conditions of about 5.0 % O2 content ATD+ sublines proliferate and differentiate ATD cysts. Cell line conversion and transdifferentiation to further vegetative/ somatic sublines are dependent of pO2 pressure. Cell cultures contain usually both cell sublines: the ATD+ subline form a minor subpopulation of only few percent while ATD-/ITD+ subline form a dominant subpopulation of >95% cells. Regarding ATD-/ ITD+ subline’s response to differentiation signals of hypoosmotic nutrient free media, it can be received in all cell cycle phases, except the S-phase. In contrast to the cyclic formation of ATD cysts from committed ATD precursor cells - exiting mitotic cell cycle at the G1/G0 exit point - ITD cyst formation is a mass differentiation process addressing to all G1/G0 and G2/M cells, indifferently if quiescent or proliferating. Changes in epigenetic configuration could be received also in G2/M, however, G2/M cells cannot be driven out of the cell cycle and finish cell division by symmetric cell division. Both identical daughter cells are capable of polyploidisation and ITD cysts formation; they enter differentiation from the post mitotically preG1state (in nutrient free media cells do not start G1 phase). Concludently, the life cycle of Entamoebae finishes by the tetranucleated mature cyst that is the mother cell of the microcell progeny. A subsequent life cycle starts by the disseminating progeny either in subcultures or in other infected hosts. Sometimes, intracolonic formed cysts lead to reinfections and amoebulae dissemination in the same host. This is a repetitive life cycle analogous with the repetitive secondary or tertiary neosis described in cancer cells by Rajamaran.30
Cancer initiation and progression are complicated processes that are regulated by a variety of cellular and signalling proteins.31 Several researchers consider that changes in tumor-associated genes (TA genes) and tumor-suppressor genes (TS) by signaling networks could be real causes of cancer initiation.32 Carcinogenesis, is the process whereby deregulated cells convert to cancer cells. Several researchers consider that in the course of human life one or more normal adult stem- and progenitor cells accumulate a sequence of intrinsic and induced/extrinsic alterations changing the antecedent cell identity.33 These changes occur at the metabolic, genetic, and epigenetic level. One of the altered/deregulated stem cells or a previously proliferating precursor cell34 converts definitively to a carcinogenic precursor cell (protoprecursor). The origin of carcinogenic cell lineage is the normal stem and early progenitor cell compartment; the cell of origin of cancer would be a transformed normal stem or progenitor cell.35−39
The cell-of-origin of cancer: a mitotic incompetent cell escaping death by mitotic bypass
There are many indications that intiation of cancer starts from a single partially deregulated cell (amitotic cell) capable of escaping cell death, by starting an atavistic cell lineage, analogous to the SPCL of protists. Due to their pre-existing capacity for self-renewal and differentiation, stem cells are attractive candidates to initiate cancer, however, this is only a supposition. The mitotic blocked cell primes the atavistic pre-carcinogenic lineage (ca-SPCL). It bypasses mitosis by a poplyploidisation path forming finally an atavistic reproductive cyst- like structure (primary aCLS) that is analogous to the ATD cysts of Entamoeba. We named this cancer initiating cell40 the protoprecursor cell. Its microcell progeny is carcinogenic and totipotent. Disseminating primary microcells starts the atavistic life cycle of cancer.
Lineage priming occurs in the early G1
According to Delorme et al.41 lineage priming is a molecular model of stem cell differentiation in which proliferating stem cells express a subset of genes associated with the differentiation pathways to which they can commit. Consequently, cells differentiate to the lineage for which they are primed. This also applies to the carcinogenic protoprecursor able to exit the mitotic cell cycle initiating the atavistic cancer cell lineage. Cell identity changes occur in a cell-cycle dependent manner. The so called “windows of changing cell identity” are key determinants for cell fate decisions.42 Stem cells are responsive to differentiation signals only during the G1 phase.42−44 Initial transcriptional changes do not require passage through mitosis however; DNA replication seems also to be related to the new epigenetic configurations that precede transcriptional transition. The relationship between the process of DNA replication and cell differentiation has not yet been completely understood. It was observed that blocking DNA replication severely impairs expression of key developmental genes.45 Waisman et al.42 states that epigenetic changes precede changes in gene expression and transcriptional profiles. Lineage priming occurs in G1 by transcriptional and epigenetic changes associated with exiting the ground state of stemness and pluripotency. Changes in gene expression begin in the same cell generation that receives differentiation signals and blocks mitosis.
Mitotic bypass occurs at the RP barrier
The canonical cell cycle exit of all eukaryotes is in the midG1 regardless of whether exiting cells are primitive protists such as Entamoeba13,27 or evolved eukaryotes such as human skeletal muscle cells.46 The G1/G0 exit point is at the end of the early G1 phase and prior restriction point RP. At this point the regular eukaryotic cell decides if it traverses the late G1 or not. Cells that do not activate genes of the late G1 phase down regulate the mechanisms of further proliferation and leave mitotic cell cycle temporarily for quiescence or terminal differentiation. There are many reasons to suppose that after mitotic blockage, changing cell identity and lineage priming, the committed protoprecursor cell leaves mitotic cell cycle at the end of the early G1 (G1/G0 exit point). In normal cells the G1 checkpoint is an active checkpoint. Most cancer cells have a defective (deficient) G1 checkpoint because of mutations in the p53 gene, deleted p53 genes, mutated Rb tumour suppressor genes47,48 or an imbalance of Cdks and cyclins.49 pRb is a retinoblastoma protein of the pocket protein family slowing cell cycle progress. In their active hypo-phosphorylated state pocket proteins prevent G1/S progression by blocking the expression of genes of S-phase initiation.50 The protoprecursor escapes death bypassing mitosis. It enters a path of polyploidisation and depolyploidisation of variable ploidy51−53 undergoing drastic genome manipulation54 involving genome rearrangements and epigenetic modifications. The protoprecursor forms finally a mature aCLS (mother polyploid cell) that segregates its multiple rearranged genome copies (WGCs) to numerous daughter cells. UGs reactivation and atavistic polyploidisation give many advantages51,55 both to the surviving mother cell as well as to its progeny. The mother cell regains reproductive function and its “transformed” progeny fits into an atavistic “immortal life cycle” that assure an indefinite number of cancer stem cell generations capable of self-renewal and differentiation (CSCs).
aCLSs mimic the reproductive innercyst cell of protists
Nobody has seen the primary aCLS of humans. Nevertheless, we assume that no relevant differences exist between primary and secondary aCLS and all primary and secondary aCLSs / native PGCCs (nPGCCs) undergo the same atavistic developmental mechanisms as protist innercyst cells. In recent years we learned that reproductive endoreplication leading to WGC ploidy is alternative to mitosis and mechanisms of atavistic reproductive ploidy are encoded in the genome of all eukaryotes.13,25−27,56 Although quite common in protists, true endopolyploidisation (≥ 8N) is rare in mammals. In humans, it occurs in embryogenesis as part of the developmental differentiating programs;57 trophoblast giant cells have polyploidisation ranges stretching between 8N and 64N. In adults, polyploidisation is an important cause of reproductive disease such as spontaneous abortions in congenital birth defects.58 There is no "spontaneous" formation of polyploids, neither in cancer nor in protists.13,27 Neither protist innercyst cells nor aCLSs are proliferating cells dividing by symmetric or asymmetric cell division. They are terminal, not further dividing cells corresponding to the ancestral terminal differentiated cell type of reproductive cell function (RTD cells). Both aCLSs and protist innercyst cells give rise to numerous microcell progeny by reductive nuclear depolyploidisation, WGC dissemination to subnuclei, subnuclei cellularization and microcell (amoebulae, Raju cells) dissemination.59−61 aCLSs and innercyst cells of protists have many analogous characteristics with each other. Similarly to protist cysts, aCLSs (PGCCs) can survive in an inactive immature state for longer time periods. Similar to protist cysts, aCLS (PGCCs) enter a state of dormancy and give rise to daughter cells that preserve stem-like properties.62 Sometimes, a single aCLS or a single amoebic cyst is sufficient to produce a metastatic tumor or amoebiasis respectively. Both structures are chemoresistant.13,27,63,64 In amoebae, unfavorable pO2 values converts the progenitor cell subline ATD+ into a vegetative/ somatic ATD-/ITD+ subline that does not form cysts in cultures. However, amoebic SPCL has alternative pathways to form a new ATD+ progenitor subline, namely by quiescent stem cells or by totipotent microcells just hatching from other cysts. We assume that the caSPCL has the same possibility. To form mature ATD cysts, regular precursor cells go through a sequence of intermediate cell stages such as immature and mature polyploids, finishing as mature innercyst cells capable of infecting new hosts or disseminating into the present host. As long as the progenitor subline proliferates, it continues to produce cysts by cyclic encystment Cysts are formed in amoebae either autonomously by the ATD+ progenitor subline (cyclic ATD encystment) or by the vegetative ATD-/ITD+ subline when amoebic cell populations are exposed to stressors (mass ITD encystment).13,27 Both differentiation pathways take place in cancer also, either as cyclic aCLS formation during cancer initiation and progression or as a mass gPGCCs formation process in cases of postgenotoxic recurrence. In the last case only a few gPGCCs remain viable. Accordingly, the molecular mechanisms leading to aCLS formation and gPGCCs recurrence are not identical. Between aCLS (PGCCs) and ATD cysts, there are also differences. In contrast to protist cysts, PGCCs can stay productive for longer periods of time.61 Amoebae cysts form a constant number of daughter cells. aCLS and gPGCCs give rise to much more progeny, the number of daughter cells is variable. aCLS formation in cancer is more hypoxic64,65 while autonomous ATD cyst formation in amoebae is more oxygenic. Hypoxia (~ 1% O2 content) favours aCLS formation, more oxygen (~ 5% O2 content) favour ATD cyst formation.13,27,28 In cancer, aCLS precursor cells need low oxygen contents for proliferation, polyploidisation and innercyst cell formation not found in regular cultures. Progenitor cells that reach the hypoxic boundary between normal and tumor necrotic tissue find optimal hypoxic conditions for fast cycling and aCLS differentiation. They form at this boundary hot spot aCLS fields. aCLSs have an actin envelope and numerous micronuclei formed by karyokinesis as described in the stock figure C030/ 3384 of the University of Pittsburgh, Cancer Institute/NCI/ Science photo library showing PGCC figure of breast cancer (http://www.sciencephoto.com/media/774447/view)The actin envelope is less resistant and more permeable than the chitin or lectin cyst walls of protists.
aCLS’ microcell progeny start the carcinogenic stem and progenitor cell lineage caSPCL
Analogous with the SPCL lineage of amoebae including the cyst producing ATD+ progenitor cell subline and the vegetative/somatic ATD-/siATD+ subline, the atavistic cancer cell lineage ca-SPCL consists of at least three basic sublines:
I. An undifferentiated multipotent stem cell line started by proliferating microcells,
II. A slow cycling progenitor subline expressing differentiation potential by asymmetric cell division (aCLS+ subline)
III. A fast proliferating vegetative/somatic subline that expresses differentiation potential only in conditions of stress (aCLS-/ siCLS+ subline); stress induces vegetative/somatic cells to mass siCLS formation.
Vegetative/somatic cancer cells do not form aCLS in cultures. Both sublines proliferate symmetrically or asymmetrically giving rise to self-renewing cells, quiescent cells and precursor cells for cyclic aCLS differentiation. In contrast to the vegetative/somatic aCLS-/ siCLS+ sublines, aCLS+ sublines proliferate in cultures slowly accelerating proliferation in conditions of hypoxia. In amoebae, low hypoxic environments (< 5.0% O2 content) transdifferentiate the ATD+ subline into a further vegetative ATD-/ ITD+ subline (transdifferentiated vegetative subline). Clones of the vegetative sublines may change their genotype, increasing their potential of invasiveness and virulence; in amoebic liver abscesses different genotypes could be isolated.13 Transidifferentiation of CSC into tumoral stromal cells is also described in cancer.66 Many authors accept today that CSCs originate from polyploid’s progeny and consider that the PGCC progeny reverts stem cell characteristics.60 The same applies to proliferating Raju cell clones30 that exhibit transient stem cell properties.61 PGCC progeny64 and the multipotent stem cells generated by it have reduced proliferation ability, similar with the primitive amoebic stem cell lines. Recently, Zhang et al.65 confirm that hatching or budding microcells generate caSPCL lineages that contain specialized sublines such as the tumorigenic progenitor cell subline. This subline gives rise, by asymmetric division and cyclic differentiation, to precursor cells forming further aCLSs. The atavistic caSPCL assure the “immortality” of the cancer life cycle: the totipotent microcells disseminated by mature aCLSs continuously form new carcinogenic lineages containing stem cells, reproductive aCLS polyploids and microcell progeny that assure the continuity of cancererous life cycles.
Neosis: a mechanisms of cyclic aCLS formation and caSPCL renewal
The term neosis was introduced in 2004 by Sundaram et al.67 as “a novel type of cell division” in addition to mitosis and meiosis. Other authors interpret neosis as a “neglected type of cell division” or as an “asymmetric cell division process”.30 Zhang et al.64 consider neosis as an atypical, unknown asymmetric cell division taking place inside PGCCs before daughter cells disseminate by budding, splitting or bursting. It was observed both in mouse fibroblasts, as well as in human p53 defficient cancer cell populations. Resulting from genotoxic treatments.30,67 In its former original sense, neosis described the reproductive process by which giant polyploids, named neosis-mother-cells (NMC), give rise to multiple viable progeny (Raju cells) capable of mitotic proliferation and invasiveness. The resting polyploids named MN/PGs are “senescent” polyploids containing a non viable genome; they die as a result of a mitotic catastrophe (MC).68−71 In fact, Sundaram et al.67 described neosis as the process of depolyploidisation, genome copy segregation and cellularisation of invasive progeny that in turn produce further generations of aCLSs and microcells. The repetitive process was named primary, secondary or tertiary neosis. But nothing is new in neosis. The authors lose sight of the asexual reproduction by polyploidisation and depolyploidisation cycles well known in protists (encystment / excystment cycle). Depolyploidization by reductive nuclear division and cellularization to multiple daughter cells is neither asymmetric cell division nor a novel type of cell division. Truly new in neosis is its unexpected appearance in human organisms and carcinogenesis, occurring not only in genotoxic crisis but also in starting carcinogenesis. The authors above do not see mitotic bypass and polyploidisation-depolyploidisation as an entity. We propose therefore to equate the term neosis with the pathway of mitotic bypass and respectively carcinogenic aCLSs and microcell formation. Accordingly, the aCLS+ progenitor subline is a neotic subline that gives rise by asymmetric cell division to neotic precursor cells which in turn forms aCLSs that disseminate neotic progeny renewing the caSPCL lineage.
Denys Wheatly (2008)60 said that finding of giant polyploid cancer cells in tissue is not difficult, but following their fate in vivo is much more tough than in vitro. We hope protist cell biology can help provide better understanding.
Permanent cancer cell lines contain both aCLS+ cells and aCLS- cells
Fei et al.72 report that human breast cancer cell lines MCF-7 and MDAMB-231 obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) are in fact heterogenous, containing cell fractions with differing resistance to hypoxia after exposure for 72 hrs to the hypoxic stressor CoCl2. The dominant fraction of regular sized cells died, while a minor cell fraction survives hypoxic stress forming giant PGCCs (aCLSs). Ten to fifteen days after exposure to CoCl2, mature PGCCs disseminate their microcell progeny in culture. Repeating the process 3-4 times, the authors select a homogenous population of polyploid mother cells and their diploid progeny. They report a mixture of 30 % PGCCs and 70 % microcells. It is evident that transitory periods of strong hypoxia such as 0.1% O2 content64 killed regular diploid cells (somatic/vegetative cells) while progenitor and PGCC-precursor cells survive. Moreover, hypoxia increases self renewal of CSCs and activates invasion- and metastasis- associated tumor genes.73−76 In our opinion both cell fractions described above belong to the dual cell system of cancer cell populations consisting of the hypoxia resistent neotic cell subline aCLSs+ (neotic subline) and hypoxia sensitive vegetative/somatic aCLS- subline. We assume that hypoxia resistant neotic subline have already anaerobe metabolism. Similar to the amoebic lineage sublines, both carcinogenic sublines live and proliferate in natural environments of different O2 content. In contrast to the neotic aCLS+ subline, the vegetative regular diploid cells of the aCLS- subline are more oxygenic.
Hypoxia favours the neotic aCLS+ subline
The anaerobe aCLS+ neotic subline increases its proliferation rate in hypoxic media by fast cycling, divides asymmetrically and gives rise to self renewing progenitor cells and committed precursor cells for aCLS differentiation. Precursor cells appear to complete aCLS differentiation even in more oxygenic environments and culture media within normal pO2 ranges. In cancer cell cultures, there is no aCLS formation by the vegetative/somatic aCLS-subline. Hypoxia changes a cell’s metabolism and plays a key role in PGCC formation.73−76 Metabolism changes induced by hypoxia are generally accepted as one of the hallmarks of cancer and changes in metabolic enzymes favour tumor formation. There is no doubt that the switch into the atavistic program needs anaerobic metabolism. Asymmetric cell division and in equal distribution of mitochondria in mitochondria poor daughter cells or mitochondria free progeny favour in cancer the switch to hypoxic metabolism. Hypoxic niches and more oxygenated perivascular zones play a key role not only in invasive intestinal Entamoebae27,28 but also in colorectal cancer.77,78 Hypoxia favours progenitor subline proliferation contributing actively to metastasis by disseminating microcells.77,64 It was also reported that hypoxia favours CSCs self renewal and promote expression of stem cell like phenotype.73−75,79 Tumor progression reflects the intrinsic properties of cancer stem and progenitor cells (CSC family) and especially tolerance to hypoxic environments.80 The authors show that cells having the potential to drive tumor growth, do not actually do so in the native tumor, because they are not in a permissive environment. In the native tumor, slowly proliferating cell sublines may be at a competitive disadvantage to more rapidly cycling cells and therefore may not contribute much to tumor growth. However, slowly proliferating sublines may form tumors after transplantation. Environmental cues from stromal cells zones can restrict the growth of cancer cells in the native tumor environment;81 the absence of such repressors permits the same cells to form tumors after transplantation.
Atavistic aCLS are rare in regular cancer cell cultures
Native PGCCs are rare in cancer cell cultures (~10-4 to 10-5).64 In our opinion, the infrequent appearance of aCLS in regular cancer cell cultures results from the different proliferation rates of the hypoxic aCLS+ subline and the more oxygenic vegetative/somatic aCLS- sublines. In cancer, progenitor cells prefer less oxygen as contained by regular cancer cell cultures. More oxygen represses progenitor self renewal leading to delayed checkpoint passage and extremely slow cycling. In Entamoeba, the ATD+ progenitor subline needs oxygen contents of about 5.0%. Oxygen contents far below or above this O2 pressure stop progenitor subline proliferation as well as the precursor cell polyploidisation and maturation needed for the checkpoint passage.28 In cancer, the vegetative/somatic aCLS- subline proliferates in regular cancer cell cultures by fast cycling; thereby, the aCLS+ subpopulation decreases while the aCLS- subpopulation increases in density. While the vegetative/somatic aCLS- subline growth was unhindered in culture media such as DMEM (Dulbecco’s modified Eagle’s medium),64,66 the neotic aCLS+ miss optimal environments for proliferation and differentiation.
Native giant polyploid cells nPGCCs as observed in vivo
As mentioned above current cancer research does not differentiate between native polyploids (nPGCCs) occurring in tissue and tumors and genotoxically induced polyploids (gPGCCs). Thereby, reader gets the wrong impression that all PGCCs or respectively all mechanisms of PGCC formation were the same. However, this is not true. Innate nPGCCs (aCLS) are found in numerous colorectal cancer forms, ovarian carcinomas, glioblastoma, non-Hodgkin lymphoma and nasopharyngeal carcinoma and human glioma (Figure 1).65,79,82−84 They utilize a highly efficient DNA replication mechanism leading to rapid malignant growth.65 Immature/ mononucleated polyploid nuclei are about three times larger than that of regular diploid tumor cells and contain numerous whole genome copies (WGC).64,65 Mature multinucleated nPGCCs (aCLSs) generate hundreds of daughter microcells by nuclear segregation, cellularisation and dissemination. It is a significant correlation between the grade of cancer disease (well, moderately and poorly differentiated cancers) and the type, location and number of nPGCCs. Zhang et al.65 show that most nPGCCs are located around necrotic tissue at the hypoxic boundary between normal and tumor tissue and believe that hypoxia favor nPGCC formation. The progeny have increased migration capacity and lead to isolated nPGCC formation that disseminates budding daughter cells in the stroma or tumor emboli. Single budding stromal nPGCCs were observed in ≥ 90% of poorly differentiated tumors, 50% of poorly differentiated and 27% of moderately differentiated colorectal cancers.65 Single stromal nPGCCs are considered to be the signal of lymph node metastasis. Usually, nPGCCs are uniformly distributed but frequently hot spot nPGCCs fields were observed. These hot spots fields occur more frequently in high grade malignant tumors and are rare in low grade tumors.
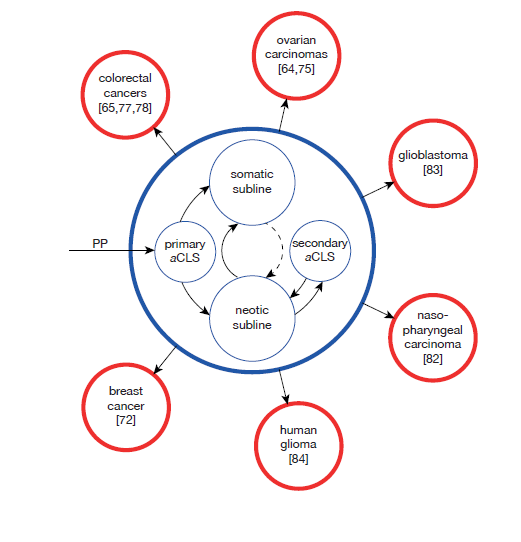
aCLS (nPGCC) is the mother cell of atavistic stem and progenitor cells
nPGCCs are tumorigenic and their microcell progeny convert to multilineages of different phenotypes as a result of activation of atavistic genes and programs.65 nPGCCs may contribute to tumor maintenance and reccurency as well as tumor radio- and chemoresistance.79 Many researchers consider nPGCCs and their microcell progeny as key players in cancer development that activate stem and progenitor cell networks.65,85 Histopathological findings substantiate the assumption that nPGCCs originate from the neotic aCLS+ subline respectively from precursor cells produced by the asymmetric dividing neotic subline that proliferates and differentiates in protist-like fashion. By this atavistic development PGCCs gain the properties of cancer stem-like cells 64,72 expressing cancer stem cell markers. In our opinion aCLSs/nPGCCs are the mother cells of atavisticly formed cancer stem and progenitor cells.
Secondary cancer cell lineages take over the basic configuration of the primary caSPCL
There is evidence that the basic configuration of carcinogenic caSPCL persists in secondary tumorigenic lineages also. nPGCCs(aCLSs) were found in colorectal cancers,65 ovarian carcinomas,64,79 glioblastoma,83 nasopharyngeal carcinoma82 and human glioma.84 Many cancer cell lines originating from solid tumors contain both somatic and neotic cells (Raju cells).72 The neotic cell subline (generating the atavistic cancer stem cell family) consists of cells proliferating in hypoxic cultures by slow cycling while the somatic subline consists of more oxigenic cells that do not survive in conditions of hypoxia.72−76 Thus, most of the atavistic cancer SPC lineages retain the basic configuration of the primary caSPCL (Figure 1) producing mother polyploid aCLSs (nPGCCs) and numerous daughter microcell progeny that restarts the caSPCL and its antagonistic aCLS+ and aCLS-/siPGCC+ sublines. In secondary tumorigenic lineages somatic vegetative cells are capable of increased cell plasticity however; none of the secondary caSPCL leaves the basic architecture of the primary caSPCL, maintained till exodus (Figure 1).
Genotoxic damage, DNA repair and checkpoint overcome
Genotoxic crisis means mitotic blockage, premature senescens and cell death but some of the damaged cells are capable of DNA repair reentering mitosis with more or less efficient repaired DNA. With other words the fate of genotoxically damaged cancer cells depends on their capacity to restore genome integrity. Genotoxic agents give rise to single or double DNA strand breaks (SSB, DSB) and DSBs are the most deletorious lesions induced by ionizing radiation. Proliferating cells of p53 deficient cancer cell lines are more sensitive to damaging agents; they enter a state of G2/M growth arrest by mitotic blockage also named stress induced premature senescense (SIPS)29,86 or accelerated cell senescense (ASC).87 Depending on the outcome of repair, damaged cancer cells may remain in mitotic blockage followed by premature apoptosis and cell death or they may continue cell cycle. In contrast to p53 proficient cells, p53 deficient cells at the G2/M check point activate a DNA damage response mechanism (DDR) that provides cells with time in order to repair genotoxic deffects. There are two different pathways to overcome the G2/M checkpoint and enter mitosis:
i. Checkpoint recovery
ii. Checkpoint adaptation.47,88,89
Checkpoint recovery occurs when cells enter mitosis with repaired DNA88 whereas checkpoint adaptation occurs when cells enter mitosis with damaged DNA.90 Inhibitors of Cdk1 may prevent cells from mitosis91,92 while Chk1 inhibitors may prevent cells from a long G2/M arrest93 respectively mitotic catastrophe and cell death. Mitotic catastrophe (MC) is the major response of cancer cell populations to moderate doses of genotoxic agents. Large parts of cancer cell population exposed to genotoxic agents dies by apoptosis or necrosis, or executes aberrant mitosis. Some of the cells escape death either by functional repair mechanisms or by better genome-surveillance mecanisms that are not yet completely understood. Swift & Golstyn47 have reported that up to 90% of cells exposed to genotoxic agents enter mitosis with damaged DNA and up to 98% of these cells die by apoptosis and necrosis, while only 2% survive as the result of checkpoint adaptation.47,89,94 In a recent paper Erenpreisa et al.87 show that genotoxic induced phenotypes such accelerated ASC can enable cancer cells to escape cell death by effective DNA repair mechanisms recovering the innate stem cell properties of CSCs. The authors assume that the reversibility of genotoxical senescense coupled to reversible polyploidy and its relation to stemness are key repair mechanisms in cancer recurrence. The authors consider that diploid- tetraploid and tetraploid-octoploid cell cycles preserve mechanisms of additional DNA repair. Depolyploidisation by micronucleation and autophagic elimination of large DNA parts or subnuclei containing damaged DNA cooperate for stemness recovery. The authors pointed out: “whether any senescing primary somatic cancer cell is capable of displaying the above features associated with senescence reversal or whether this only applies to cancer stem cells (CSCs) and how the recovery finally occurs is still largely unclear”.
The dual response of cancer cell populations to genotoxic insults
As described in previous chapters most cancer cell lines in cultures form distinct subpopulations originating from the antagonistic aCLS+ and aCLS- sublines. These cell fractions are genotypically, phenotypically and metabolically different however they possess both aCLS differentiation potential. While the minor more hypoxic aCLS+ subline proliferates in hypoxic environments by fast cycling and asymmetric division forming mature polyploids, the dominant more oxygenic vegetative/somatic subpopulation aCLS-does not express this potential in cultures and does not form polyploids. The minor neotic aCLS+ subpopulation includes cells of the cancer stem cell family that express typical cancer cell markers.72,95 The CSC family contains the neotic cell stages begining with progenitor and precursor cells and ending with mature aCLSs and totipotent microcells that start in turn the caSPCL. In contrast to the more differentiated slow cycling neotic aCLS+ subpopulation, the dominant aCLS-subpopulation consists of regular diploid cancer cells that proliferate by fast cycling and dye in conditions of hypoxia. Both subpopulations respond differently to genotoxic agents and irradiation. In contrast to slow proliferating tumor stem and progenitor cells that are less damaged by genotoxic agents,96 tumor proliferative cells are more damaged: they die or enter mitotic catastrophe. Moreover, cells exhibit differential genotoxic sensitivity in different phases of the cell cycle, with cells in mitosis being most sensitive to DNA damaging agents and cells in late S-phase being most resistant.97,98 Cell cycle progression favors or disfavors the fate of the damaged cells and their ability to survive.
Check point deficiency and genotoxic crisis
Cancer cells response to genotoxic agents mostly depends on their p53 cell status. In contrast to the most p53 deficient cells, proficient p53+ WT cells arrested at the G1/G0 check point, escape genotoxic crisis and remain viable. They secrete growth promoting factors and give rise finally to stem cell-like progeny. On the other side, more of 50% of human cancer lineages have p53 deficient or null genotype and the lack of G1/G0 arrest and absence of p53 does not exclude propagation of surviving cell descendants after genotoxic treatment. The most important checkpoint in response to DNA damage in many cancer cells is the G2/M checkpoint.47 TP53 is the most commonly mutated gene in human cancer and TP53 mutations that lead to loss of wild-type p53 activity in different tumor types; according to Muller and Vousden99 this contributes to cancer progression and more aggressive tumor profiles functioning as a negative inhibitor over any remaining wild-type p53. There is an assumption that p53 deficiency could be a consequence of carcinogenesis and not a condition sine qua non of transient senescence, DNA synthesis, endopolyploidization and micronucleation. Mansila et al.100 propose that polyploidisation by chemotherapeutics may be also induced in p53+ proficient cancer cells such as the HCT116 (p53+/+) human colon carcinoma cell line. Some cells can overcome defective mitosis by arrest in the ensuing G1 phase; this arrest is considered to be p53 dependent.101,102 On the other side a mitotic blocker such as vinblastine, leads to cell cycle stoppage with PGCC formation from the G1 state.103 This is evidence that PGCC formation may occur from both G1/G0 and G2/M blocked cells.61 It can be conclude that formation of endopolyploid cells is not an exclusive characteristic of cells lacking wild-type p53; it can be also induced by low doses of doxorubicin in p53+/+ cells. p53 and p21WAF1 protein levels decrease after treatment with drugs104−109 or as a result of alterations in the phosphorilation of Chk1.110
Somatic cancer cells transdifferentiate post-genotoxically to neotic cells
Regarding the p53 deficient cancer cell populations in genotoxic crisis Erenpreisa et al.87 consider that “stress induced senescent cancer cells retain the proliferation potential through induced polyploidy coupled to active autophagy”. This is correct, but we do not forget that the aCLS differentiation potential is genomically encoded not only in the neotic aCLS+ fraction but also in the vegetative/somatic aCLS-/siCLS+ subpopulation that does not express differentiation potential during growth however, after genotoxic treatment a few vegetative/somatic cells express differentiation potential. How does one interpret that?
Genotoxically stressed p53 cancer cells arrest to the beginning of the genotoxic crisis in a tetraploid G1 state (mitotic blockage) forming different cell fractions. Some of the damaged cells (MC fraction) repair partially the DSB strand breaks. As a result they enter mitosis with damaged DNA (checkpoint adaptation) and dye by mitotic catastrophe (MC); Most of the genotoxically damaged cells remain in a state of mitotic blockage (premature senescense) and dye by premature apoptosis (Apoptotic cell fraction). Only a minor cell fraction escapes apoptotic death conversing to the reproductive/neotic pathway (Mitosis bypassing fraction). The reason of this differentiation is unknown. Asynchronous culture progression at the time of irradiation may be a possible factor. Thus, the amitotic tetraploid G1cell decide if somatic damaged cells may switch into the alternative neotic pathway forming gPGCCs and corresponding microcell progeny (gCSCs). Cells switching to the neotic cell state express the atavistic differentiation potential, hidden in the somatic cancer cell genome. The decision to enter the polyploidisation-depolyploidisation pathway bypassing mitosis is taken in the tetraploid G1 state.
Atavistic cancer stem cells in cultures and radioresistance
Maintaining the common characteristics of stemness several researchers have demonstrated that many types of tumors and cancer cell lines contain CSCs that express stem cell surface markers and are tumorigenic in xenotransplants.111,112 Cell-surface markers, such as CD133 and CD144 (cluster of differentiation marker) are potential prognostic factors for treatment outcomes. It was also shown that CSCs in cultures are resistant to anti-cancer drugs and irradiation. Several multi-drug resistant (MDR) genes and multi-drug resistant proteins (MRP) such as the breast cancer resistant protein 1 BCRP1 contribute to drug resistance.111,113,114 Wang et al.112 consider that cancer cell lines grown for a long time in vitro may not truly reflect all biological features of primary CSC of primary cultures due to adaptations and genetic alterations taking place in the hyperoxic conditions of growth occurring in long-term culturing. Studying primary cancer cell lines isolated by tissue resection from freshly isolated tumors, authors observed that primary CSCs are more resistant to conventional chemotherapy and radiotherapy than populations of more differentiated (older) cancer cell lines. Primary CSCs (intrinsic CSCs) may remain in primary and residual tumors after treatment contributing to cancer recurrence and spreading.112 According to many published reports, the main mechanisms of radioresistance of CSCs are the robust DNA-repair capacity and enhanced reactive oxygen species (ROS) defenses. CSCs are virtually resistant to radiation and cytotoxic chemotherapy, and may contribute to genotoxic resistance and tumor recurrence.115 CSCs proliferate slowly, and are found largely in the G0 phase of the cell cycle, for extended periods of time.115,116 Factors such as the microenvironment and autophagy also confer increased radioresistance to CSCs.115,117−119 Numerous studies have shown that intrinsic cancer stem cells (CSCs) - that represent a small subpool of the heterogenous cancer cell population in hetero-geneous tumors - are responsible for radioresistance.120−128 We assume, this also applies to the neotic CSC family.
The present paper corroborates the idea that human cancer originates by atavistic mechanisms encoded in human’s dark genome. We suppose, a mitoticly incompetent protoprecursor cell escapes death bypassing mitosis (neotic bypass). It enters an alternative cyle of ancestal reproductive differentiation inherrited from the common last eukaryotic ancestor. According to the atavistic cancer cell theory it is a process of MGs downregulation and upregulation of UGs. The protoprecursor starts an atavistic polyploidisation process ending by depolyploidisation and formation of totipotent microcells capable to initiate a multilined stem and progenitor cell lineage (Figure 2). The atavistic cancer cell lineage caSPCL is structured similarly to the stem and progenitor cell lineage SPCL of intestinal amoebae. Cancer cell populations in culture and permanent cell lines contains two antagonistic sublines; The hypoxic aCLS+ neotic subline proliferates by asymmetric cell division forming precursor cells for aCLS differentiation, analogous to the ATD cyst formation in amoebae arising from the ATD+ subline (cyclic encystment). The more oxygenic vegetative/somatic cancer cell subline aCLS-/siCLS+ is analogous to the vegetative/somatic ATD-/ITD+ subline of amoebae; both sublines do not produce cysts or cysts-like structures in cultures, but possess differentiation potential and express it in conditions of stress. In p53 defficient cancer cell lines genotoxic stress induces most cells of the vegetative/ somatic aCLS-/siCLS to apoptosis and death or to mitotic catastrophe. Few somatic cells are blocked in the tetraploid G1 state. They escape amitotic death switching to neotic transdifferentiation. By this way they form new atavistic stem cell families (aCSCs). Genotoxic treatments cannot completely destroy the atavistic lineage capable of recurrence (Figure 2).

Dr. Ekaterina Erenpreisa played a decisive role in starting this work. Several years ago she was enthusiastic about the reproductive polyploidy occurring in pathogenic amoebae and suggested to me to write a comparative paper on the analogies existing between the primitive stem and progenitor cell system of amoebae and cancer cells. I thank Dr. Erenpreisa for the constructive exchange of ideas and support. I express my gratitude to Dr. Dennis Thomas (native English speaker) for reading the manuscript and improve my English.
Authors declare there is no conflict of interest in publishing the article.

©2018 Niculescu. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.