MOJ
eISSN: 2641-9300


Review Article Volume 1 Issue 1
Research at own home, Japan
Correspondence: Tsuneo Ishida, Research at own home, Japan
Received: December 01, 2017 | Published: December 19, 2017
Citation: Ishida T. Anticancer activities of silver ions in cancer and tumor cells and DNA damages by Ag + - DNA base-pairs reactions. MOJ Tumor Res. 2017;1(1):8 -16. DOI: 10.15406/mojtr.2017.01.00003
Bactericide mechanisms are thought to become clear that bacteriolysis and destruction of bacterial cell wall occur due to imbalance of bacterial cell wall peptidoglycan (PGN) syntheses and PGN autolysins. Therefore, Ag ion induced PGN hydrolases promote activations of the autolysins for amidases against Gram-positive S.aureus and for amidase and carboxypeptidase-transpeptidase against Gram-negative E.coli. Silver nanoparticles (AgNPs) can defeat more osteosarcoma cells than the tumor suppressor of p53 is used for human cancers by triggering mitochondrial stress and apoptotic elimination of cancer cells. Silver nitrate showed inhibitory effects against adenocarcinomic alvcolar basal epithelial (A549) cells in a dose- and time-dependent manner for 24, 48, and 72h and induced apoptosis. The autophagy induced by AgNPs promoted cell survival, as inhibition of autophagy by either chemical inhibitors or enhanced AgNPs-elicited cancer cell killing. The AgNPs can inhibit cancer cell growth and angiogenesis. ROS-related mechanism and angiogenesis involved in stem cell differentiation, as well as ROS-induced anti-angiogenesis reactions could be a promising capable of exploiting. AgNPs induced apoptosis was found dependent on the overproduction of ROS and DNA damage-mediated p53 phosphorylation to advance HePG-2 cell apoptosis. Recently, results indicated the potential of AgNPs to promote adipogenic differentiation of human MSCs (hMSCs) in an ROS-dependent mechanism. The AgNPs showed a significant reduction in the migration of MCF-7 cells, which the AgNPs not only inhibited cancer cell proliferation through induction of apoptosis and as well inhibited the cancer cell migration. Lastly, nano particles- induced cancer cell C-terminal hydrolases lead to the apoptosis and the necrosis, and enhance cancer and tumor cell deaths. DNA damages due to silver-complex formation within DNA base-pairs G≡C, A=T occur in cytoplasm of cancer cell. Silver atom is twofold coordinated by two N atoms, and N-Ag+-N complex of linear type is formed in DNA base pairs. In ground state, O-Ag+-N, N-Ag+-N, N-Ag+-O complexes of twofold coordinated linear type may be formed at the G≡C pair, and whereas, N-Ag+-O, N-Ag+-N complexes of twofold coordinated linear type may be formed at the A =T pair.
AgNPs, Silver Nanoparticles; AgC, Colloidal Silver; AgPyNPs, Nanosized Silver(II) Pyridoxine Complexes; BAX, Bcl-2-Associated X Protein; BEAS-2B, Bronchial Epithelial 2B; Coco-2, Colon Cancer Cell 2; CFU, Colony Forming Units; ENPs, Engineered Nanoparticles; E.coli, Escherichia Coli; GBM. Glioblastoma Multiforme; GI, Gastrointestinal; hMSCs, Human Mesenchymal Stem Cells; HIFs, Hypoxia-Inducible Factor; IC50, Half Maximal Inhibitory Concentration; PGN, Peptidoglycan; PC-3, Prostate Cancer 3; MCF-7, Michigan Cancer Foundation-7; MIC, Minimum Inhibitory Concentration; NADPH, Nicotinamide Adenine Dinucleotide Phosphate; ROS, Reactive Oxygen Species; S.aureus, Staphylococcus Aureus; SCCs, Silver N-Heterocyclic Carbene Complexes; SNPs, Silver Nanoparticles; TME,Tumor Micro- Environment; VEGF, Vascular Endothelial Growth Factor
silver nanoparticles, autophagy, reactive oxygen species, cancer, tumor, cells, angiogenesis, cancer cell hydrolase, dna damage, ag+-dna base pairs interaction
Silver has long been known to have antimicrobial properties and were used until chemical antibiotics came into vogue. Metallic silver and most inorganic silver compounds ionize in moisture, body fluids and secretions to release biologically active Ag+, which the silver ions have been used for the characteristics of toxicology and pharmacology as antimicrobial agent.1 Nanosilver particles have been widely used in a range of biomedical application, including diagnosis, treatment, drug delivery, medical device coating, and for personal health care. Due to recent advances in nanotechnology, it is now possible to produce silver at nanoscale. In addition to their potential electronic and transparent conductor applications, the emergence of nanosilver materials in antimicrobial consumer goods and medical products is driving the growth of the nanosilver market.2 Among the different nanosilver preparation, silver nanoparticles(AgNPs) can be synthesized in a variety of ways, each with its own advantages and disadvantages. The most common methods of AgNPs synthesis are through chemical reduction of silver salt (AgNO3) using a reducing agent (sodium borohydride).2 During chemical reduction, silver ion Ag+ receives an electron from the reducing agent and reverts to its metallic form Ag0 eventually clustering to form AgNPs. AgNPs are cytotoxic to cancer cells and possess excellent potential as an antitumor agent, in which AgNPs induce cytotoxicity, generate reactive oxygen species (ROS), and cause mitochondrial damage to human cells. Toxic effects indifferent cell types depend on the interactions and distribution patterns of the nanoparticles.3 The size of nanoparticles influences the binding and activation of membrane receptors and subsequent protein expression in cancer cells.3 AgNPs of 12 nm size caused chromosomal aberration and DNA damage, and induced proliferation arrest in cell lines of zebrafish indicating that AgNPs must be investigated for their potential teratogenic effects in humans.4 AgNPs have applications in many industries including biomedicine, pharmaceuticals, biotechnology, nanomedicine, and instrumentation as antibacterial agents, drug delivery agents, biosensors, and environmental sensors due to their unique properties. In this review, firstly antibacterial activities and the bactericide mechanisms of Ag+ ion solution were investigated, in which antibacterial mechanisms for the actions of Ag+ ion solution by bacteriolyses and destructions of bacterial cell walls were clarified. Secondly, the anti-cancer activities of silver nanoparticles (AgNPs) are generally investigated, in which the interactions of anticancer activity of silver (I) ions occur in each regions of cancer and tumor cell development. Finally, it shed light on that AgNPs play various significant roles of cancer promotion and progression, tumor formation and growth, in which lead to apoptosis and necrosis, anti-tumor activity, anti-inflammation and differentiation, anti-proliferation, anti-angiogenesis, important function of autophagy and ROS production, anti-invasion and anti-metastasis, and the damages by Ag+-DNA base-pairs interactions.
MIC and CFU
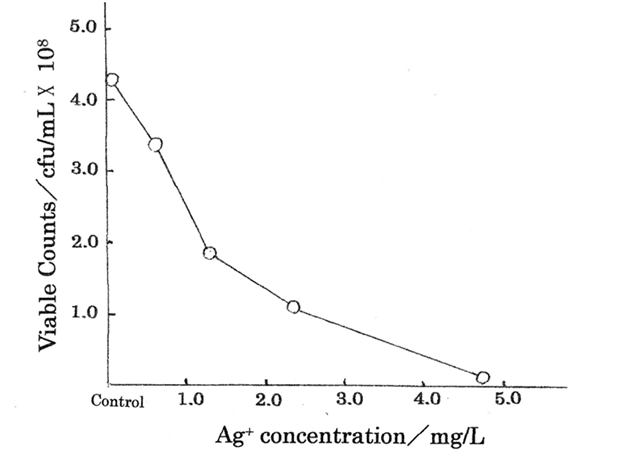
Ag+ ions are important as antibacterial agents for bactericide action in bacterial cells. Table 1 shows the bacteriostasis as disinfection agent inhibiting the bacteria growth and multiplying organism of Ag+ ion, in which minimum inhibitory concentration, MIC= 4.70 mg/L was obtained for Ag+ ion concentration range of 0.59~4.70 mg/L against E.coli. The killing curve of Ag+ ions is shown in Figure 1 (measurement’s error= ±6%), indicating that the relationship between Ag+ ion concentration(mg/L) and viable colony counts(CFU/mL) has been expressed, in which killing effects for the silver (I) ions appear sufficiently.5 Killing mechanisms of Ag+ ion solutions against bacteria are outlined below.
Ag+Ion solution sgent and |
Ag+ concentrations (mg/l) |
|
|
|
|
4.7 |
2.34 |
1.17 |
0.59 |
Control |
|
MIC (4.70 mg/L) |
- |
+ |
+ |
+ |
+ |
CFU (x 108 CFU/mL) |
1.0x106 |
1.1x108 |
1.8x108 |
3.3x108 |
4.2x108 |
Table 1 MIC measurement of commercial Ag+ ion solution agents and viable cell counts (CFU/mL) against
Antibacterial mechanism by Ag+ ion solution
Bactericide mechanisms are thought to become clear that bacteriolysis and destruction of bacterial cell wall occur due to imbalance of bacterial cell wall peptidoglycan(PGN) syntheses and PGN autolysins. Therefore, Ag ion induced PGN hydrolases promote activations of the autolysins for amidases against Gram-positive S.aureus and for amidase and carboxypeptidase-transpeptidase against Gram-negative E.coli.6 Therefore, it may be important problems that the considerations of these antibacterial mechanisms are whether can be applied appreciably for anti-cancer activity of silver ion solution against cancer and tumor cells.
Silver nanoparticle in cancer and tumor cells
Silver nanoparticles(AgNPs) that are created and integrated nanotechnology with biotechnology and medicine, have been widely used as a novel therapeutic agent extending its use as antibacterial, antifungal, anti-viral and anti-inflammatory agents.7 Cell damages also by AgNPs may be due to loss of cell membrane integrity, apoptosis and oxidative stress. Bio-synthesized AgNPs will open a new direction towards various biomedical applications in near future. The water soluble AgNPs of size 9~32 nm with a face-centered cubic structure showed dose-dependent cytotoxicity against prostate cancer(PC-3) cells, in which explored a potential anticancer application of AgNPs for prostate cancer therapy.8 AgNPs prepared in culture medium and sonicated, are cytotoxic and genotoxic also for endothelial progenitors, in particular for endothelial colony-forming cells, in which participate to angiogenesis.9 Thus, these AgNPs are toxic for microvascular endothelial cells and for endothelial precursors, indicating that they could be exploited to contrast angiogenesis. Polyelectrolyte complex beads containing silver nanoparticles (beads/AgNPs) significantly inhibited the growth of colon cancer cells (Caco-2).10 This study shows that the beads/AgNPs suppressed the oxidation of Ag0 at low pH=2.0, and promoted the gradual release of Ag+ when subjected to slightly basic conditions (pH=7.4), in which was intrinsically linked to cytotoxic action on the Caco-2 cells, opening new perspective biomaterials with anti-tumor property. Furthermore, core-shell/polymeric nanoparticles11 and AgNPs/Cisplatin12 provide significant enhanced cytotoxic effect and antitumor efficacy against breast cancer and ovarian cancer respectively, in which AgNPs-based combination therapy may aid in formulating novel and more effective cancer therapeutics. The other, recently magnetic nanoparticles (MNPs) can heat up tumor tissues and induce killing of cells under external AC magnetic field. However, magnetic nanoparticles hyperthermia requires high concentration of MNPs that are injected into the tumor, in order to obtain clinically needed thermal dose because of the complicated heat transfer and the limited heat quality of MNPs.13 Colloidal silver (AgC) also induces could be useful as an antiproliferation, inducing an impairment of tumoral growth.14 The AgC had dose-dependent cytotoxic effect in MCF-7 breast cancer cells through induction of apoptosis, and significantly increased SOD activities.15
Homeostasis and apoptosis
Apoptosis or programmed cell death causes death of 50~70 billion cells every day in the average human adults. This process is called homeostasis and takes place for self-renewal of tissues, such as skin, gut, and bone marrow. The disruption of homeostasis can lead to cancer and therefore, apoptotic genes have been focused at cancer treatment. Silver nanoparticles (AgNPs) caused cancer cell apoptosis, cell cycle arrest and decreased viability of colon cancer cells.16 AgNPs can defeat more osteosarcoma cells than the tumor suppressor of p53 is used for human cancers by triggering mitochondrial stress and apoptotic elimination of cancer cells.17 AgNPs also induced p53-mediated apoptosis in bronchial epithelial (BEAS-2B) cells, in which AgNPs induced oxidative stress, activated the p53 protein, and triggered p53-mediated apoptosis.18 Engineered nanoparticles (ENPs) also induced apoptosis may aid in the design of effective cancer.19 Characteristics of ENPs, such as sizes, shape, forms, charges and surface modifications are all seen to play a role in determining their toxicity in target cells. Silver nitrate showed inhibitory effects against adenocarcinomic alvcolar basal epithelial (A549) cells in a dose- and time-dependent manner for 24,48, and 72h and induced apoptosis.20 The half-maximal inhibitory concentration IC50 value of silver nitrate also induced apoptosis according to immunocytochemical assays for 72h. The silver nitrate may be a suitable therapeutic agent against lung cancer. Apoptotic efficacy of biogenic AgNPs against breast cancer MCF-7 cell lines showed significant cytotoxic activity with IC50 value 3.04μg/mL compared to that of standard cisplatin.21 The nanosilver showed excellent apoptosis rate due to their smaller size and spherical morphology, in which AgNPs contribute a novel and alternate approach in cancer therapy.
Anti-tumor activities
Several silver N-heterocyclic carbene complexes (SCCs) have been synthesized and tested for their anticancer activities that are active against ovarian, breast, melanoma, colon, renal, bladder, and prostate human cancer lines.22 The results of silver complexes SCCs provide effective inhibition of cancer cell growth against various cell lines, in which to eliminate the challenge of silver precipitating out in chlorinated solutions, SCCs have been successfully encapsulated into nanoparticles. AgNPs that biologically synthesized, have an efficacy of an anti-tumor agent using Dalton’s lymphoma ascites (DLA) cell lines in vitro and in vivo.23 The antitumor properties of AgNPs may be a cost-effective alternative in the treatment of cancer and angiogenesis-related disorders. AgNPs synthesized by a microbiological method, were tested for their antitumor activity against MCF-7 and T47D cancer cells and MCF10-A normal breast cell line.24 These test results indicated in cell viability, apoptosis induction, and endo-cytosis activity of those cell lines that the effects of the biosynthesized AgNPs were directly related with the endocytosis activity. Moreover, AgNPs had higher inhibition efficacy in tumor lines than in normal lines of breast cells, which is due to the higher endocytic activity of tumor cells compared to normal cells. Green synthesis of silver nanoparticles (SNP) opens a new path to kill and prevent various infectious diseases and tumor.25 The activities of SNP were checked with human pathogens, plant pathogen and marine pathogen and studied the scavenging effect and anticancer properties against MCF-7 cell lines, in which the SNP appears extremely fast, cost efficient, eco-friendly and alternative for conventional methods of SNP synthesis to promote the usage of these nanoparticles in medicinal application.
Inflammation and differentiation
Inflammation has been linked to any number of diseases including prostate cancer, in which inflammatory signaling promotes tumorigenesis. Chronic inflammation has been shown to promote the initiation and progression of diverse malignancies by inducing genetic and epigenetic alterations. Bacterial infection-induced prostatitis results in microenvironmental changes that enhance the differentiation of prostate basal cells into luminal cells, the limiting step in transformation of basal cells. This differentiation process accelerates tumorigenesis starting in basal cells, supporting a unique role for inflammation.26,27 The inflammation promotes prostate differentiation. While cancer can arise from mutant stem cells, in which does not explain why tissues without defined stem cell populations are susceptible to inflammation-driven tumorigenesis. However, it is said that chronic inflammatory diseases, such as colitis and pancreatitis, predispose to gastrointestinal(GI) adenocarcinoma by programming differentiated cells, and it is proposed that dysregulation of cell fate may be a novel rate-limiting step of tumorigenesis, by discovering recently connections between inflammation and loss of cell differentiation.28 AgNPs caused differentially regulated cytotoxicity and induced neuronal differentiation of F9 tetratocarcinoma stem cells in a concentration-dependent manner.29 AgNPs can be used for differentiation therapy, along with chemotherapeutic agents, for improving cancer treatment by targeting specific chemotherapy-resistant cells within a tumor, and more the molecular mechanism of apoptosis and differentiation in stem cells could also help in developing new strategies for cancer stem cell (CSC) therapies. Ag+ ion and nanoparticulate silver on the differentiation of human mesenchymal stem cells (hMSCs) were investigated,30 in which resulting that ionic or nanoparticulate silver attenuates the adipogenic and osteogenic differentiation of hMSCs even at non-toxic concentration. Both AgNPs and Ag+ caused transcriptomic changes that could potentially exert an adverse effect on development.31 Although transcriptomic responses to AgNPs and Ag+ were substantially similar, AgNPs exerted specific effects on embryonic stem cells (ESCs) due to their nanosized particulate form. AgNPs induce blood brain barrier (BBB) inflammation and neurotoxicity,32 in which AgNPs may interact with the cerebral microvasculature producing a proinflammatory cascade, these events may further induce brain inflammation and neurotoxicity. Further, anti-inflammatory action of the nanocrystalline silver is still unclear, but the anti-inflammatory effect of nanocrystalline Ag and the molecular mechanism have becoming clarified.33 AgNPs of 3-5 nm size can enter in mouse neural cells to induce pro-inflammatory cytokine secretion and increase Aβ amyloid deposition in response to the changes of gene expression in inflammatory response, oxidative stress, and Aβ degradation, in which AgNPs-induced neuroinflammatory response and Aβ deposition might evolve the progress of neurodegenerative disorders.24 Nanosized silver(II) pyridoxine complex (AgPyNPs) exhibit a better immunological response with low cytotoxicity compared to silver nanoparticles, which is a powerful beneficial feature to heal wounds and infection-related issues.35 Pyridoxine possesses antioxidant and cell proliferation activity. The AgPyNPs showed less cytotoxicity compared with AgNPs by producing a small amount of ROS in RAW264.7 macrophage cells and generate a more active inflammatory response and a more vigorous immune protection. Thus, AgPyNPs exhibit better therapeutic ability compared to silver nanoparticles for treating and healing wounds.
Proliferation
The interactions of AgNPs with the lung fibroblasts (IMR-90) and glioblastoma cells (U251) were investigated.36 The electron micrograph revealed a uniform intracellular distribution of AgNPs both in cytoplasm and nucleus, in which AgNPs treated cells exhibited chromosome instability and mitotic arrest in human cells [36]. The major route of nanoparticle uptake is through clathrin dependent endocytosis and micropinocytosis. Exposure of AgNPs resulted in chromosomal abnormalities, inhibition of proliferation, observed as failure to form colonies, and absence of recovery selectively in cancer cells, which add new hopes for preventing cancer cell metastasis.36 Anti-proliferation activities of AgNPs of about 30 nm size were studied against lung cancer cells, which have great promise as antitumor agents.37 Further, AgNPs have been shown to function largely against cultured glioblastoma multiform (GBM),38 which the antiproliferative properties of silver nanoparticles overwhelm proapoptotic ones on the cytotoxic effect of AgNPs and on tumor cells should be considered. The other, silver-based nanoparticles applied alone reduce the viability of oral squamous cell carcinoma cells while the natural compound berberine diminishes their antiproliferative effect.39 AgNPs at low doses reduce the proliferation and viability of oral squamous cell carcinoma cells (SCC). SCC-25 cells are susceptible to damage from AgNPs-induced stress, which can be regulated by the natural alkaloid berberine, suggesting that AgNPs may be used in a chemoprevention/chemotherapy by argumentation action of anti-cancer drugs.39
Autophagy and cell death
Autophagy is a biological process whereby large molecules and damaged organelles in the cytoplasm are degraded. Tumor cells are usually in hypoxic state and are associated with nutrient and growth factor deficiencies, which are activators of autophagy. Induction of autophagy may be a form of self-preservation mechanism for tumor cells to survive in the hypoxic, highly acidic, and/ or toxic environment. Autophagy have a lysosomal degradation process which is key for the regulation of the turnover of long-lived or damaged proteins and organelles and which promotes cell survival during nutrient deprivation or other microenvironmental stresses. Autophagy is a multi-step process characterized by nucleation, elongation and autophagosome and autolysosome formation, and is tightly regulated by a limited number of highly conserved genes called autophagy regulators.40 A key step during this process is formation of autophagosomes. Autophagosomes mainly consist of cytoplasmic contents and damaged organelles, such as the mitochondria. Defective autophagy is correlated with diverse pathologies, including neurodegeneration, liver, heart and muscle diseases, ageing, inflammation and cancer. AgNPs of 58.8±1.7 nm nano-size were shown to be cytotoxic to cancer cells and possess excellent potential as an antitumor agent, in which the autophagy induced by AgNPs was characterized by enhanced autophagosome formation, normal cargo degradation, and no disruption of lysosomal function. The autophagy induced by AgNPs promoted cell survival, as inhibition of autophagy by either chemical inhibitors or enhanced AgNPs-elicited cancer cell killing.41 AgNPs of about 10 nm size synthesized by Bacillus flexus promoted cell death in lung cancer cells, as cancer cells are slightly more acidic than normal cells,42 in which the effect of hypoxia on AgNPs-induced apoptosis was that hypoxia-inducible factor (HIF)-1αinhibited AgNPs-induced mitochondria-mediated apoptosis by regulating autophagic flux through the regulation of tumor cell. The combination of salinomycin (Sal) and AgNPs had more pronounced effect on cytotoxicity and expression of apoptotic genes and also significantly induced the accumulation of autophagolysosomes, which was associated with mitochondrial dysfunction and loss of cell viability.43 Both Sal and AgNPs induce massive autophagy, which in turn leads to mitochondrial dysfunction and cell death. This finding suggests that Sal plus AgNPs-treated cells experienced significantly higher toxicity than cells treated with Sal or AgNPs only.43 The strong synergistic interaction between Sal and AgNPs increased the therapeutic potential and demonstrated the relevant targeted therapy, in which the combination of AgNPs and Sal enhances apoptosis and autophagy in ovarian cancer cells. The other, the protein-capped bAgNPs using biogenic-NPs in therapeutic application were found to be way more cytotoxic than bare or chemically synthesized AgNPs, which might be attributed to the enhanced internalization facilitated by the presence of protein coat or due to controlled release of Ag ions.44 This mycosynthesized bAgNPs induce cytotoxicity through elevation of reactive oxygen species (ROS) levels and induction of apoptosis, in which simultaneous induction of autophagy tends to inhibit apoptosis by restricting enhanced ROS production and cancer cell death.
Angiogenesis and cancer cell growth
Angiogenesis, which is the formation of new blood vessels from pre-existing ones, is regulated by the balance of many stimulating and inhibiting factors. While physiological angiogenesis plays a crucial role in several pathological conditions, such as tumor growth, invasion and metastasis, the disruption of this control causes the proliferation of a network of blood vessels penetrating into cancerous growth.45 Various components of the angiogenesis process are necessary to promote new vessel formation in health and diseases. Vascular endothelial growth factor (VEGF) and fibroblast growth factor-2 (FGF-2) are important promoters of this process. These pro-angiogenesis growth factors are able to degrade the basement membrane, which the degradation allows cells to migrate and proliferate at the cleared-away area and differentiate into lumen-containing vessels. AgNPs reduced (AgNO3) with diaminopyridinyl (DAP)-derivatized heparin (HP) exhibited effective inhibition of basic (FGF-2)-induced angiogenesis, with an enhanced anti-angiogenesis efficacy with the conjugation to DAPHP (P≦0.01) as compared to glucose conjugation. DAPHP-reduced silver nanoparticles have potential in pathological angiogenesis accelerated disorders such as cancer and inflammatory diseases.45 Next, AgNPs of 16.5±1.2 nm size synthesized using Saliva officinalis on chick chorioalantoic membrane (CAM) could be of immense use in medicine anti-angiogenic properties, in which the CAM structure, after treatment with toxic dosages of nanoparticles appeared to be clustered with a few cellular extensions and vessel formation, and cell-spreading patterns were restricted compared to the control groups.46 In the CAM model, silver nanoparticles have dose-dependent cytotoxic effects on endothelial cells, inhibited blood vessel formation and showed its inhibitory effect on angiogenesis. Further, a hypoxic tumor microenvironment (TMA) is widespread in solid tumor. It is a result of the disrupted balance between supply and consumption of cellular O2, owing to fast tumor growth and irregular vasculature. Hypoxia and high proliferation of cancer cells produce excessive reactive oxygen species, such as hydrogen peroxide, promote angiogenesis, and lead to metastasis of cancer cells.47 The vascular network delivers oxygen O2 and nutrients to all cells within the body. O2 availability serves as a primary regulator of this complex organ. Most transcriptional responses to low O2 are mediated by hypoxia-inducible factors (HIFs), highly conserved transcription factors that control the expression of numerous angiogenic, metabolic, and cell cycle genes.47 AgNPs of about 10 nm size inhibited the activation of a HIF-dependent reporter construct after the cells were exposed hypoxic conditions or treated with cobalt chloride, a hypoxia mimetic agent. The AgPNs inhibit HIF-1 function by attenuating its protein accumulation and downstream target expression, owing to cytotoxicity and angiogenesis induced by AgNPs.48 The AgNPs can inhibit cancer cell growth and angiogenesis.
Ag ion induced ROS production in angiogenesis
ROS during cell signaling and homeostasis are reactive species of molecular oxygen. The ROS constitute a pool of oxidative species including superoxide anion ・O2-, hydroxyl radical ・OH, hydrogen peroxide H2O2, singlet oxygen 1O2, and hypochlorous acid HOCl. Reactive oxygen species (ROS) are natural byproducts of cellular oxidative metabolism and play important roles in modulation of cell survival, cell death, differentiation, cell signaling, and inflammation-related factor production. ROS are generated intrinsically or extrinsically within the cell.49 Molecular oxygen generates ・O2-, the primary ROS via one-electron reduction catalyzed by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase.50 Further reduction of oxygen may either lead to H2O2 or ・OH via Haber-Weiss and metal-catalyzed Fenton reactions.51 Treatment with 5 nm AgNPs reduced nuclear factor erythroid 2-like expression in both cell types without affecting its activation at the early time point after AgNPs treatment. Increased ROS production was detected 1 h after 5 nm AgNPs treatment, and lactate release was restored in the presence of an ROS scavenger. Thus, 5 nm AgNPs affect glucose metabolism in hepatoma by producing ROS.52 AgNPs have been shown to provide a novel approach to overcome tumors, especially those of hepatocarcinoma. Therefore, this study was carried out to estimate the effect of AgNPs on proliferation and activation of ROS-mediated signaling pathway on human hepatocellular carcinoma HePG-2 cells.53 It was resulted that 2 nm AgNPs markedly inhibited the proliferation of HePG-2 cells through induction of apoptosis with caspase-3 activation, in which AgNPs induced apoptosis was found dependent on the overproduction of ROS and DNA damage-mediated p53 phosphorylation to advance HePG-2 cell apoptosis. Recently, results indicated the potential of AgNPs to promote adipogenic differentiation of human MSCs (hMSCs) in an ROS-dependent mechanism.54 ROS-related mechanism and angiogenesis involved in stem cell differentiation, as well as ROS-induced anti-angiogenesis reactions could be a promising capable of exploiting.54
Inhibition of invasion and metastasis
Cancer cells invade other tissues either by moving collectively as epithelial sheets, detached clusters, and as single cells via mesenchymal or amoeboid cell types. The tumor cell migration and invasion are critical steps in the cancer metastatic cascade. Tumor cells interact activity with the tumor microenvironment (TME) with many physiological events such as cancer cell, in which the tumor cell-TME interactions become important.40 The other hand, metastasis is one of the most distinct, nevertheless complicated biological phenomena in cancer. It is still a formidable problem not only in malignant tumors, but also a lethal diagnosis often encountered in later stage tumors including breast cancer. Therefore, prevention or inhibition of metastasis has an important clinical application for prolonging and enhancing the quality of life. Cell migration plays a central role in metastasis, which is composed of multiple steps. The effect of AgNPs on cancer cell migration in the breast adenocarcinoma MCF-7 cell line was investigated.55 MCF-7 cells treated with various concentrations of AgNPs of about 48 nm size showed a dose-dependent increase in cell inhibition. The IC50 value of the AgNPs was 42.5μg/mL. The AgNPs showed a significant reduction in the migration of MCF-7 cells, which the AgNPs not only inhibited cancer cell proliferation through induction of apoptosis and as well inhibited the cancer cell migration. Similarly, 30nm AgNPs coated with citrate or poly (ethylene glycol) (PEG) were used to assess the influence of coating on the effects produced on a hepatoma cell line (HepG2), namely, in terms of viability, apoptosis, apoptotic related genes, cell cycle and cyclins gene expression.56 At the concentrations used (11 and 5μg/mL corresponding to IC50 and IC10 levels, respectively) the amount of cells undergoing apoptosis was not significant and the apoptotic related genes BCI.2 (anti-apoptotic gene) and BAX (pro-apoptotic gene) were both down regulated.56 Thus, this work has shown that citrate- and PEG-coated AgNPs impact on HepG2 apoptotic gene expression, cell cycle dynamics and cyclin regulation in similar way, in which the decrease of proliferation may be due to both cytostatic effect and/or necrotic events.
As the main compartment for intracellular degradation and subsequent recycling of cellular constituents, the lysosomes receive both hetero- and autophagic cargo, which in the degradative lumen of this organelle find their final destination. The degradation is carried out by a number of acid hydrolases such as phosphatase, nucleases, glycosidases, peptidases, sulfatases, and lipases capable of digesting all major cellular macromolecules.57 The physical disruption, including leakage of lysosomal hydrolases into the cytosol, led to programmed cell death and necrosis. Numerous regulatory proteins direct the addition of ubiquitin to lysine residues on target proteins, and there are countered by an army of deubiquitinating enzymes. BRCA1-associated Protein-1(Bap1) helps to control cell proliferation by resulting HCF-1 protein levels and by associating with gene involved in the G1-S transition.58 Therefore, silver ions induced carboxy-terminal hydrolase activation may be promoted the apoptosis and the necrosis of cancer cells. Ubiquitin C-terminal hydrolase-L1 (UCH-L1) increases cellular ROS levels and promote tumor invasion, in which silencing UCH-L1, as well as inhibition of H2O2 generation by catalase, a NOX inhibitor, suppressed the migration potential of B16F cells, indicating that UCH-L1 promotes cell migration by up-regulating H2O2 generation59 The other, gold nano rod induced apoptosis specifically in cancer cells by affecting lysosome and mitochondria.60 Lysosomes are membrane-bound organelles containing that function in the degradation of macromolecules delivered via the endocytic, phagocytic, and autophagic pathways,61 in which AgNPs induced cell death was increased by bafilomycin Al treatment, and the perturbation of lysosomal pH by AgNP exposure may play a role in AgNP agglomeration and subsequent cellular damage in cancer cell. Elongated nanoparticle aggregates and generated hundreds of pN to dramatically damage the plasma and lysosomal membranes, whereas the physical disruption, including leakage of lysosomal hydrolases into the cytosol, led to programmed cell death and necrosis.62 This work provides a novel strategy of designing magnetic nanomedicines for mechanical destruction of cancer cells. Comparing with bacterial PGN autolysins, the research of cancer cell hydrolases will become important as significant advancement for cancer and tumor cells in future.
Ag+ ion induced generations of ROS and hydrogen peroxide H2O2 in tumor cells damage DNA in tumor, in which formation of DNA damage resulting from a release of catalytic Ag+ ion to DNA with generation of ・OH radicals, and by reaction of H2O2 with the metal produces the strand breaks in DNA as well as DNA base modifications and deoxyribose fragmentation. The sites of action tending to bind purine base adenine(A), guanine(G) and pyrimidine base cytosine(C), thymine(T) of nucleic acid bases for individual metals can be presumed,63 in which are depending on acid dissociation constant pKa. It has been found that in aqueous solution coordination of Ag+ to the N3 and N1 sites of purine rings is pH dependent and coordination to N3 may diminish as pH of the solution increases64 in which silver ion-nitrogen affinity65 and silver nanocluster66 are considered. According to the theory, it is shown in Figure 2 that is represented to substituting of Ag+ ions into hydrogen bonds in DNA base-pairing G≡C and A=T pairs respectively. Thus, it may be considered that DNA damages due to silver-complex formation within DNA base-pairs G≡C, A=T occur in cytoplasm of cancer cell. Silver atom is twofold coordinated by two N atoms, and N-Ag+-N complex of linear type is formed in DNA base pairs (center of Figure 2). In ground state,67 O-Ag+-N, N-Ag+-N, N-Ag+-O twofold coordinated linear type may be formed at the G≡C pair, and whereas, N-Ag+-O, N-Ag+-N complexes of twofold coordinated linear type may be formed at the A =T pair (right side of Figure 2). A: T base pairs are less stable than G: C base pairs in Ag+-DNA.68
The summary of above-mentioned results is represented in Table 2 that the anti-cancer activities of Ag+ ions act for cancer prevention, promotion, progression, proliferation, invasion, and metastasis against cancer and tumor cells.
Ag+ Ion solution |
Progression and growth |
||||
|---|---|---|---|---|---|
|
Ag+ |
Prevent ion |
Promotion |
Progression |
Proliferation and |
Metastasis angiogenesis |
Oncogenes |
Invasive growth |
||||
Carcinogenesis |
Tumorigenesisinitiation, |
Malignant |
Cell migration |
Transendothelial migration |
|
|
|
|
|
Ag+ |
|
O2-,-OH, H2O2 |
O2-,-OH, H2O2 |
O2-,-OH, H2O2 |
O2-,-OH, H2O2 |
||
Topical silver |
AgNPs; |
||||
S |
IC50= 42.5 μg/mL anti-metastasis. |
||||
Sal+ AgNPs inhibit malignant cell |
Sal+ AgNPs with autophagy inhibit proliferation |
PEG coated AgNPs inhibit metastasis |
|||
AgNPs induced ROS anti-angionesesis |
AgNPs induced ROS anti-angiogenesis |
AgNPs induced ROS anti-angiogenesis |
|||
Degradation by hydrolase |
Degradation by hydrolase |
Degradation by hydrolase |
|||
AgNPs/ Cisplatin combination therapeutics |
AgNPs of size 10 nm minute. |
||||
Table 2 Anti-cancer activity reaction of Ag+ ions for the initiation, progression, proliferation, invasion, and metastasis against cancer and tumor cells
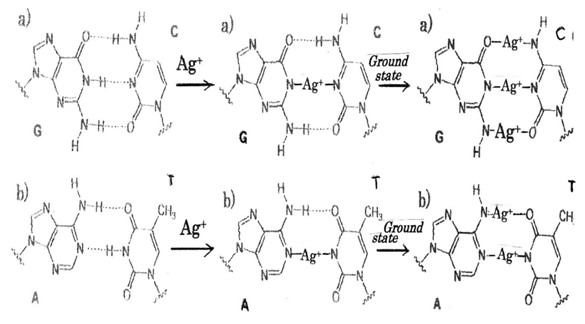
Bactericide mechanisms are thought to become clear that bacteriolysis and destruction of bacterial cell wall occur due to imbalance of bacterial cell wall peptidoglycan (PGN) syntheses and PGN autolysins. Therefore, Ag ion induced PGN hydrolases promote activations of the autolysins for amidases against Gram-positive S.aureus and for amidase and carboxypeptidase-transpeptidase against Gram-negative E.coli. Silver nanoparticles (AgNPs) are toxic for microvascular endothelial cells and for endothelial precursors, indicating that they could be exploited to contrast angiogenesis. AgNPs caused cancer cell apoptosis, cell cycle arrest and decreased viability of colon cancer cells. AgNPs can defeat more osteosarcoma cells than the tumor suppressor of p53 is used for human cancers by triggering mitochondrial stress and apoptotic elimination of cancer cells. AgNPs also induced p53-mediated apoptosis in bronchial epithelial (BEAS-2B) cells, in which AgNPs induced oxidative stress, activated the p53 protein, and triggered p53-mediated apoptosis. Engineered nanoparticles (ENPs) also induced apoptosis may aid in the design of effective cancer. Silver nitrate showed inhibitory effects against adenocarcinomic alvcolar basal epithelial (A549) cells in a dose- and time-dependent manner for 24, 48, and 72h and induced apoptosis. The autophagy induced by AgNPs promoted cell survival, as inhibition of autophagy by either chemical inhibitors or enhanced AgNPs-elicited cancer cell killing. The AgNPs can inhibit cancer cell growth and angiogenesis. ROS-related mechanism and angiogenesis involved in stem cell differentiation, as well as ROS-induced anti-angiogenesis reactions could be a promising capable of exploiting. AgNPs induced apoptosis was found dependent on the overproduction of ROS and DNA damage-mediated p53 phosphorylation to advance HePG-2 cell apoptosis. Recently, results indicated the potential of AgNPs to promote adipogenic differentiation of human MSCs(hMSCs) in an ROS-dependent mechanism. The AgNPs showed a significant reduction in the migration of MCF-7 cells, which the AgNPs not only inhibited cancer cell proliferation through induction of apoptosis and as well inhibited the cancer cell migration. Lastly, nanoparticles- induced cancer cell C-terminal hydrolases lead to the apoptosis and the necrosis, and enhance cancer and tumor cell deaths. DNA damages due to silver-complex formation within DNA base-pairs G≡C, A=T occur in cytoplasm of cancer cell. Silver atom is twofold coordinated by two N atoms, and N-Ag+-N complex of linear type is formed in DNA base pairs. In ground state, O-Ag+-N, N-Ag+-N, N-Ag+-O complexes of twofold coordinated linear type may be formed at the G≡C pair, and whereas, N-Ag+-O, N-Ag+-N complexes of twofold coordinated linear type may be formed at the A =T pair. A: T base pairs are less stable than G: C base pairs in Ag-DNA.
None.
Authors declare there is no conflict of interest in publishing the article.

©2017 Ishida. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.