MOJ
eISSN: 2641-9300


Research Article Volume 1 Issue 5
1NN Blokhin Russian Cancer Research Center, Russia
2Department of Oncohematology, Moscow Clinical Scientific and Practical Center, Russia
3NI Pirogov Russian National Research Medical University, Russia
Correspondence: Galina A Dudina, Candidate of Medical Sciences, Senior Researcher, Department of Oncohematology, Moscow Clinical Scientific and Practical Center, Russia
Received: August 21, 2018 | Published: September 12, 2018
Citation: Kalitin NN, Dudina GA, Semochkin SV, et al. Expression of the VEGF-A/VEGFR1/VEGFR2 signature genes: potential new diagnostic and prognostic molecular markers in patients with myelodysplastic syndromes (MDS). MOJ Tumor Res. 2018;1(5):161-165. DOI: 10.15406/mojtr.2018.01.00036
Study objective: To evaluate the expression of the VEGF-A/VEGFR1/VEGFR2 signature genes as potential new diagnostic and prognostic molecular markers in patients with myelodysplastic syndromes (MDS).
Materials and methods: The expression of VEGF-A, VEGFR1, and VEGFR2 genes in mononuclear cell fractions obtained from 15 healthy volunteers and 24 primary patients with MDS was studied by real-time polymerase chain reaction (real-time PCR).
Results: Expression of all three genes was detected in the studied population. The average expression of the VEGF-A gene (p<0.0001) was highest in the general group of patients, while the VEGFR1 gene expression in the patients prevailed over the expression of the VEGFR2 gene (p<0.001). The mean values of the VEGF-A, VEGFR1, and VEGFR2 gene expression were significantly higher in the MDS group compared to the control group. The VEGF-A gene expression levels were positively correlated with the percentages of blast cells in the patients (p<0.05) and the VEGFR1 gene expression levels (p<0.05), but not that of VEGFR2. Only the expression of the VEGFR1 gene was significantly negatively correlated with the overall survival of patients (r= -0.5, p<0.05). Patients with prognostically unfavorable levels of blast cells (>5%) demonstrated a tendency toward an increased average expression of VEGF-A and VEGFR1, with a reduced expression of the VEGFR2 gene. The median overall survival was significantly (p<0.001) different in patients with blast cell levels > 5% vs. <5% (16 months vs. 60 months, respectively).
Conclusion: The expression of the VEGF-A/VEGFR1/VEGFR2 signature genes can be used as a molecular diagnostic and prognostic marker in MDS patients.
Keywords: myelodysplastic syndromes, VEGF-A, VEGFR1, VEGFR2 gene expression
Myelodysplastic syndromes (MDS) are a heterogeneous group of clonal disorders of the hematopoietic system that arise from hematopoietic stem cells. The initial link in the pathogenesis of MDS is genetic changes (point mutations, chromosomal aberrations) accumulating in pluripotent cells of the erythroid, granulocyte, and megakaryocyte hematopoietic lineages, ultimately leading to their damage and transformation.1,2 The maturation in one, two, or three hematopoietic lineages is disrupted, which manifests in changes in the morphological properties and functional activity of the hematopoietic cells of these lineages.3 These reorganizations in the hematopoietic system result in one-, two-, or three-lineage cytopenia in the peripheral blood in patients with MDS against the background of hypercellularity (more rarely normo- or hypocellularity) of the bone marrow.4 In addition, a characteristic feature of different MDS variants is steadily progressing multilineage dysplasia of ineffective hematopoiesis with a subsequent increase in the blast cell count in the bone marrow and transformation into acute leukemia.5 Multiplication of the blast cell pool in MDS is controlled by a variety of autocrine and paracrine signaling pathways that are able to significantly modulate their malignant phenotype and lead to disease progression.6 Some of these signals may be associated with increased expression of the vascular endothelial growth factor VEGF-A and its two receptors, VEGFR1 and VEGFR2. It has been demonstrated that the VEGF-A growth factor and VEGFR1 and VEGFR2 receptors are not only involved in the regulation of angiogenesis and neoangiogenesis under normal conditions and in malignant transformation but also promote the proliferation of cells in many solid and nonsolid tumors.7,8 In particular, the pathogenetic significance of this receptor system has been established for multiple myeloma and human lymphomas.9,10
On the other hand, the significance of the expression of the VEGF-A growth factor and the VEGFR1 and VEGFR2 receptors as possible molecular links in the pathogenesis of MDS and their role as potential diagnostic and prognostic factors in MDS has been little researched. Aguayo A et al.,11 did not identify any prognostic value of the serum VEGF-A level in patients with MDS but established that the same parameter is predictive in patients with acute myeloid leukemia. At the same time, Verstovsek et al.,12 demonstrated that high VEGF-A expression levels were negatively correlated with survival in patients with both MDS and acute myeloid leukemia, whereas the expression of the VEGFR1 and VEGFR2 receptors did not have prognostic significance in the studied groups of patients. The study aim is to investigate the VEGF-A, VEGFR1, and VEGFR2 gene expression in primary patients with MDS, to compare the obtained data on the VEGF-A, VEGFR1, and VEGFR2 expression in the group of MDS patients with the control group, and to evaluate the possible clinical and pathogenetic value of the VEGF-A/VEGFR1/VEGFR2 signature in MDS.
A group of 15 healthy volunteers (6 men and 9 women) and 24 patients (15 women and 9 men) with newly diagnosed MDS were studied. Informed consent was obtained from all patients and healthy volunteers prior to the study initiation. The age of patients ranged between 51 and 85 years (median age: 71.5 years). Among the patients enrolled in this study, three subjects were diagnosed with refractory anemia (RA); five, with MDS associated with isolated del(5q); one, with refractory anemia with ring sideroblasts (RARS); six, with refractory cytopenia with multilineage dysplasia (RCMD); two, with type 1 refractory anemia with excess blasts (RAEB-1); and seven, with type 2 RAEB (RAEB-2). The patients were classified into the abovementioned MDS variants in accordance with the WHO classification (2008).13 Using the International Prognostic Scoring System (IPSS) of MDS, the patients were stratified as follows: seven at low risk, seven at intermediate risk 1, three at intermediate risk 2, and seven at high risk.14 The study materials were peripheral blood samples obtained from healthy volunteers and bone marrow aspirates taken from patients. These biological materials were then separated in a Ficoll density gradient (PanEco, Russia) to obtain a mononuclear cell fraction from which the total RNA was isolated using TRIzol Reagent (MRC, USA). All the procedures have been previously described in detail.15 To synthesize cDNA, 1 μg of total RNA, 1 μL of "random 6" degenerate hexamer primers (Syntol, Russia), 2.5 mM of dNTP mixture (MBI Fermentas, Lithuania), 0.4 U of RNAse inhibitor (MBI Fermentas, Lithuania), 2 U of the M-MuLVplus reverse transcriptase (Syntol, Russia) were used for the reverse transcription. The mixture volume was 25 μL. The synthesis was performed in a Tertsik thermocycler (DNA-technology, Russia) at 42 °C for 50 min with preliminary incubation for 10 min at 25 °C. The reaction was stopped by heating to 70 °C for 10 minutes.
The real-time PCR was performed in a Bio-Rad CFX amplification systems (Bio-Rad, USA) using the EvaGreen intercalating dye (BIOTIUM, USA) as follows: 95 °C for 5 min for 1 cycle and then 39 cycles: denaturation 95 °C, 20 s; annealing of primers 59 °С, 25 s; synthesis 72 °C, 20 s. Each sample was examined in triplicate. The nucleotide sequences of the primers are listed in Table 1. The expression of the RPL27 gene of the 60S ribosome subunit was used to normalize the data. The relative level of expression of each gene was determined using the formula 2ΔCt: [∆Ct=Ct (RPL27) – Ct (of the studied gene), where Ct is the minimum gene threshold cycle in the exponential phase of the amplification curve]. The statistical processing of the results was performed using the GraphPad Prism 5.02 software. The results are reported as M±EM or m (where M is the arithmetic mean of the sample, EM is the standard error of the mean, and m is the median). The statistical significance was determined using Student's t-test at p<0.05. Correlations were determined using the Pearson (r) or Spearman (R) correlation. The overall survival curves were calculated using the Kaplan-Meier estimator, and a statistically significant difference in survival was determined using the Log-Rank test.
Gene |
Sequence |
VEGF-A |
5’- AGGGCAGAATCATCACGAAGT- 3’(for) |
5’- AGGGCTTCGATTGGATGGCA-3’ (rev) |
|
VEGFR1 |
5’- TTTGCCTGAAATGGTGAGTAAGG- 3’ (for) |
5’- TGGTTTGCTTGAGCTGTGTTC-3’(rev) |
|
VEGFR2 |
5’- GGCCCAATAATCAGAGTGGCA- 3’(for) |
5’- CCAGTGTCATTTCCGATCACTTT -3’(rev) |
|
RPL27 |
5’- ACCGCTACCCCCGCAAAGTG - 3’(for) |
|
5’- CCCGTCGGGCCTTGCGTTTA - 3’(rev) |
Table 1 Nucleotide sequences of primers used in real-time PCR
Note: for is the sequence of the forward primer; rev is the sequence of the reverse primer.
We determined the expression levels of the VEGF-A gene as well as the VEGFR1 and VEGFR2 receptor genes in mononuclear cell fractions obtained from 15 healthy volunteers and 24 patients with MDS. Our data showed that the individual expression levels of VEGF-A, VEGFR1, and VEGFR2 in the group of healthy individuals demonstrated little variability, while in the MDS group the expression of the investigated genes was within a broader range of values (Figure 1). As can be seen in Figure 1, among the VEGF-A/VEGFR1/VEGFR2 signature genes, the expression of the VEGF-A growth factor was higher than that of the VEGFR1 and VEGFR2 genes in 100% of the healthy volunteers enrolled in the study and in 22 of 24 patients (91.6%). The individual expression values of the VEGFR2 receptor gene were the lowest both in the control group (0.000023 –0.0002 ± 0.00001) and in the patients (0.00003 – 0.016 ± 0.00066). A comparison of the mean VEGF-A gene expression in these two groups demonstrated that the VEGF-A expression was significantly higher—by 4.54 times (p<0.0001)—in the patients compared to the control group (Figure 2A). When comparing the mean expression levels of the VEGFR1 gene, we also identified higher VEGFR1 expression (by 1.96 times, p<0.05) in the general group of patients in contrast to the group of healthy volunteers (Figure 2B). The most pronounced difference in the expression between the control group and the MDS group was observed for the VEGFR2 receptor gene: the average VEGFR2 expression level in patients was 29.7 times higher (p <0.05) than that in healthy individuals (Figure 2C). At the same time, the additive level of the VEGFR1 gene expression in the general group of patients was 2.36 times higher (p<0.01) than that of the VEGFR2 gene (Figure 3).
Since blast cells are one of the key factors in the pathogenesis of MDS, and they to a significant extent determine its clinical course, we analyzed whether the expression of the VEGF-A, VEGFR1, and VEGFR2 genes changed in the patients from the general group who had different percentages of blast cells. We did not find a statistically significant difference in the expression of the investigated genes depending on the number of blast cells (Figure 4). The mean level of the VEGF-A gene expression in Subgroup 2 (> 5% of blast cells) was only 21% higher than in Subgroup 1 (<5% of blast cells). The expression of the VEGFR1 gene was also insignificantly elevated (by 19.6%) in Subgroup 2 compared to Subgroup 1. The mean expression of the VEGFR2 receptor gene in Subgroup 2 was, on the contrary, decreased by 67% relative to Subgroup 1. Despite the absence of a difference in the mean expression of VEGF-A, VEGFR1, and VEGFR2 in Subgroups 1 and 2, which differ in the blast cell counts, we determined that these subgroups were significantly distinct in the median overall survival. In patients with blast cell count <5% (Subgroup 1), the median overall survival was more than 3 times higher (p<0.01) compared to patients in Subgroup 2 (Figure 5). In addition, we identified a positive correlation between the blast cell count and the level of the VEGF-A gene expression (r=0.49, p <0.05), but not that of the VEGFR1 and VEGFR2 genes. Moreover, we determined that the expression of the VEGF-A growth factor gene was positively correlated (r=0.46) with the expression of the VEGFR1 receptor gene, and this was statistically significant (p<0.05). For the VEGFR2 receptor gene, there was no such correlation with the VEGF-A expression (r=0.36, p=0.08).
A correlation analysis of the relationship between the overall survival rates in 24 patients enrolled in this study and the expression levels of the VEGF-A, VEGFR1, and VEGFR2 genes demonstrated that the expression of all three genes was negatively correlated with the overall survival of the patients but was statistically significant (r = -0.5; p<0.05) only for the VEGFR1 gene. Indeed, when only individual VEGFR1 gene expression values in each patient that deviated from the median (m=0.0047) were adopted as a differentiating criterion, thus dividing the general group of patients into Subgroup 1 (VEGFR1 expression <m) and Subgroup 2 (VEGFR1 expression > m), we identified a significant difference between the overall survival curves of these two subgroups (p<0.05). Therefore, the median overall survival for Subgroup 1 was 94 months, and for Subgroup 2, 29 months (Figure 6).

Figure 1 The differential expression of the VEGF-A/VEGFR1/VEGFR2 signature genes in the control group and in the group of MDS patients.
Note: N is the control group; T is the group of patients with MDS.

Figure 2 The additive mean expression levels of the VEGF-A growth factor (a) and its receptors VEGFR1 (b) and VEGFR2 (c) in the control group and the group of patients with MDS.
Note: N is the control group; T is the group of patients with MDS; *=p<0.05; ***=p<0.0001.
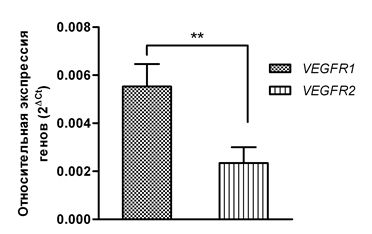
Figure 3 A comparisons of the average levels of the VEGFR1 and VEGFR2 gene expression in the studied patients.
Note: **=p<0.01.
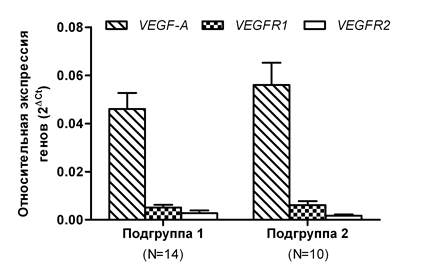
Figure 4 Changes in the expression of the VEGF-A/VEGFR1/VEGFR2 signature genes, depending on the blast cell count in the bone marrow of the patients.
Note: Subgroup 1 includes patients with blast cells <5%; Subgroup 2 includes patients with blast cells >5%; N is the number of patients.
In recent decades, a significant number of studies in the field of experimental oncology has been devoted to the development of new approaches and algorithms in the diagnosis of various malignant neoplasms. The changes in various molecular markers investigated in them allow identifying the characteristic differences between the pools of neoplastic and normal cells, which is important for diagnostic purposes. On the other hand, changes that are specific only for the cancer cell pool from which a certain malignant tumor is formed can be correlated with its clinically important indicators; therefore, these changes can be significant in terms of the prognostic value. In this study, we investigated the hypothetical marker value of the expression of the VEGF-A vascular endothelial growth factor gene and genes of its receptors, VEGFR1 and VEGFR2, as diagnostic and prognostic factors for patients with different MDS types. Our findings indicate that the expression of all VEGF-A/VEGFR1/VEGFR2 signature genes was significantly higher in the MDS group than in the control group. It is known that activation of signaling pathways triggered by the VEGF‑A ligand and the VEGFR1 and VEGFR2 receptors can induce various mitogenic and antiapoptotic cellular programs and not be limited to the process of neovascularization.16 At the same time, new growth of blood vessels with the participation of VEGF-A and its VEGFR1 and VEGFR2 receptors has also been proven crucial in stimulating tumor growth and metastatic spread.17 In this regard, we hypothesized that overexpression of the VEGF-A/VEGFR1/VEGFR2 signature genes may indicate their important pathogenetic role in MDS associated with the functioning of the VEGF-A–VEGFR1 and/or VEGF-A–VEGFR2 signaling pathways.
A comparison of the total average expression levels of VEGFR1 and VEGFR2 demonstrated that the average expression of the VEGFR1 gene was significantly higher than the expression of the VEGFR2 gene in the general group of patients. This probably indicates that the VEGF-A–VEGFR1 system, but not the VEGF-A–VEGFR2, is the dominant one in the patients we studied, and it is this system that may be responsible for transmission of both angiogenic and proproliferative signals capable of inducing disease progression. Our findings are partially in line with a number of studies that discovered a negative prognostic value of increased expression of the VEGF-A growth factor in MDS.12,18 The blast cell count in the bone marrow and peripheral blood of MDS patients is not just an important classification criterion, but it also has a crucial predictive significance.4 We identified virtually no difference in the average expression of the VEGF-A and VEGFR1 genes between Subgroups 1 and 2 that differed in the blast cell counts (<5% or >5%, respectively), whereas changes in the VEGFR2 gene expression between these subgroups were more pronounced. We also noted that a decrease in the averageVEGFR2 expression in Subgroup 2 relative to Subgroup 1 was observed along with a slight increase in the expression of the VEGF-A and VEGFR1 genes in Subgroup 2. Moreover, we identified statistically significant positive correlations between the expression of VEGF-A and the blast cell percentages in the examined patients as well as the expression levels of the VEGF-A and VEGFR1 genes, but not between VEGF-A and VEGFR2.
These patterns of changed expression of the VEGF-A/VEGFR1/VEGFR2 signature genes depending on the blast cell count in patients with MDS, in fact, support our previous assumption of the predominant significance of the VEGF-A growth factor and primarily its VEGFR1 receptor in the malignant evolution of MDS. F. Wimazal et al.19 also found a positive correlation between the VEGF-A gene expression and the percentage of immature myeloid cells (blasts and monocyte progenitors), while higher levels of the VEGF-A protein were detected in patients with RAEB, RAEB in transformation into acute leukemia (RAEB-t), and patients with chronic myelomonocytic leukemia (CMML) compared to the control group. A comparative analysis of the median overall survival in Subgroups 1 and 2 demonstrated a significantly higher value of this parameter in Subgroup 1 (blasts <5%) vs. Subgroup 2 (blasts >5%). It is important that only the expression of the VEGFR1 gene had a statistically significant negative correlation with the survival of all 24 patients enrolled in this study, but not the expression of VEGF-A and VEGFR2. We have also previously demonstrated on a group of 19 primary patients with multiple myeloma (MM) that increased coexpression of the VEGF-A and VEGFR1 genes was observed in patients with significantly higher levels of bone marrow infiltration by plasma cells, a greater concentration of tumor paraprotein in the blood serum, and a lower median overall survival.20
Therefore, the observed differences in the additive mean VEGF-A, VEGFR1, and VEGFR2 gene expression levels between the control group and the MDS group suggest that the expression of the VEGF-A/VEGFR1/VEGFR2 signature can be used as an independent diagnostic test in patients with different types of MDS. The data presented in this article on the correlation between the expression of the VEGF-A/VEGFR1/VEGFR2 signature genes as well as the percentages of blast cells in the bone marrow and the overall survival of the studied patients allow considering the quantitative levels of expression, primarily of the VEGF-A and VEGFR1 genes alone and together, as new candidate prognostic markers for patients with MDS.
None
The author declares there is no conflict of interest.

©2018 Kalitin, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.