MOJ
eISSN: 2379-6294


Research Article Volume 1 Issue 2
University of Ontario Institute of Technology, Canada
Correspondence: John Guchardi, University of Ontario Institute of Technology, 2000, Simcoe Street North, Oshawa, Ontario, L1H7K4, Canada,Tel 19057218668 x2838
Received: November 05, 2014 | Published: May 8, 2015
Citation: Krause R, Guchardi J, Holdway D. Use of immunofluorescence with frozen sections for the detection of vitellogenin year-2 class natural population rainbow trout-a preliminary investigation. MOJ Toxicol. 2015;1(2):43–50. DOI: 10.15406/mojt.2015.01.00007
Vitellogenin (VTG) is an egg yolk precursor protein produced in the liver in response to estrogen and is taken up by the oocytes of rainbow trout (Oncorhynchus mykiss) by receptor-mediated endocytosis. It is a useful biomarker which can be detected in blood plasma using an enzyme-linked-immunosorbent assay (ELISA) by using primary and secondary antibodies to detect the protein. This study utilized 12 juvenile male and female rainbow trout, to develop and test a new method as a hybrid of frozen section histology and ELISA to directly detect VTG within gonad tissue using immunofluorescence (IF). Frozen sections of gonads fluoresced bright yellow within Medium Stage IV cells, indicating the presence of VTG within the developing oocytes. Paraffin and frozen histology sections were also compared for efficacy and quality. The two methods produced similar results, however frozen sectioning was faster and the tissue was better preserved when viewed under the microscope. Paraffin provided excellent color contrast, but the tissue exhibited more damage when compared to the frozen sections. Use of frozen sections to examine morphology and IF to detect VTG in rainbow trout gonadal cells proved to be effective for analyzing VTG at the tissue level.
Keywords: rainbow trout, biomarker, vitellogenin, immunohistochemistry, cryo-section, histology, immunofluorescence
ELISA, enzyme-linked-immunosorbent assay; IF, immunofluorescence; VTG, vitellogenin; EDC, endocrine disrupting chemicals; IHC, immuno histochemistry; GSI, gonadal somatic index; NBF, neutral buffered formalin; RTDW, room temperature distilled water; PBS, phosphate buffered saline; BSA, bovine serum albumin; FITC, fluorescein isothiocyanate
As a result of stimulation of the hypothalamic-pituitary axis in fish, gonadotropins act on target organs, such as the gonads, to produce sex hormones. Estrogens are secreted from the ovaries and circulate in the blood until they bind to receptors in target organs. In the liver, estradiol receptors mediate the production of vitellogenin (VTG), a phospho lipoprotein complex required for oocyte maturation and the egg yolk precursor protein.1 From the liver, VTG travels in the blood back to the ovary where it is absorbed into oocytes through receptor-mediated endocytosis.1 VTG absorption accounts for more than 80% of oocyte growth resulting in a 1000-fold increase in volume of the cell.2 A more detailed description of the pathway and its regulation can be found in Arcand-Hoy & Benson.1 Although both sexes preserve the ability to produce the protein VTG, it is not normally present at significant levels in the blood or gonads of male fish during their life cycle.3 However, males cannot dispose of VTG readily, thus levels can remain high in blood plasma of males longer than in females.3 If an organic compound is close enough in structure and function to the endogenous estrogens present in sexually mature females, it can act as an estrogen mimic or blocker and can lead to disruption of VTG levels.4 Unlike other reproductive biomarker proteins, VTG is not produced by any other mechanism except induction due to estrogens, making it very specific.5 This makes VTG useful in bioassays to determine the phenotypic sex of fish, the developmental stage, or to evaluate potential feminizing effects of exposure to endocrine disrupting chemicals (EDCs) by measuring plasma levels/presence in tissues.6,7 VTG can be detected using an enzyme-linked-Immunosorbent assay (ELISA).8 It uses a micro plate with primary and secondary antibodies linked to an enzyme which produces a colorimetric reaction in proportion to the concentration of protein antigen within the sample. This procedure can be used for detection of any number of proteins with complementary antibodies and is used in medical and environmental labs to detect antigen presence in the blood and in the organs of organisms. Knowing the presence and concentration of VTG in the blood plasma provides information about the stage of reproductive maturity, but does not provide information about the morphology of the gonad. Histology is one approach used to investigate how VTG levels affect the fish at the tissue level.
The most popular method for traditional histology is paraffin wax sectioning.9,10 To detect a protein biomarker within histological sections, Immunohistochemistry (IHC) or immunofluorescence (IF) is utilized. Although paraffin histology has been trusted for decades to preserve tissue long term, it may not be the best method for IHC and IF.11 The preservation of epitopes on protein antigens is essential for antibody recognition. Fixatives such as formalin and ethanol dehydration steps in the embedding process cause protein cross-linking and disruption of intermolecular interactions, which affect antibody binding in fluorescence staining.12 The method of frozen tissue sectioning or Cryo sectioning is becoming routine for IF and IHC. This is a quick way to embed and mount histological sections and does not require tissue fixation. It maintains the integrity of proteins and enzymes within the tissue so that labeled antibodies can bind and be visualized by fluorescent or enzyme markers.13 IHC and IF are more specific for tagging antigens than enzyme staining or general structural staining.14 It is also possible to co-stain the same sub cellular structure with multiple fluorochromes, providing another level of specificity.12 IHC and IF have been applied to analyses of biomarkers in numerous ways but rarely are the same method and antibodies used to identify the presence of the same protein in the blood and the tissue. VTG is found in multiple locations in the body, therefore, in order to utilize VTG as a biomarker, identifying its location directly in tissue and blood is important. This is where ELISA meets histology; the detection method used for VTG in the blood can be applied to locating it in the tissue. This study had two objectives. First to optimize the frozen sectioning method to compare it to traditional paraffin histology for efficacy, difficulty and results. Secondly, to develop a novel method using a hybrid of both ELISA and immunofluorescence that would allow for direct detection of the VTG protein location within cells of the gonad. By comparing with blood VTG levels, this method would help complete the picture of VTG analysis at the morphological level.
Dissection and freezing
Twelve sub-adult, two year old rainbow trout (Oncorhynchus mykiss), seven (7) females and five (5) males, obtained from the local hatchery, were lab acclimated for 18 months without exposure to any chemicals. Fish were anesthetized using tricaine methane sulfonate (100mg/L MS-222) for all procedures. Morphometric data such as total weight, gonad weight, length and sex were recorded to calculate physiological indices such as condition factor.15
Where K=the Condition Factor, W=is the weight of the fish in grams (g), and L=is the fork length of the fish in millimeters (mm) and
.
Blood samples were taken using caudal puncture with 6ml heparin coated vacationers. The vacationers were stored on ice at 4°C for approximately 1 hour until centrifugation at 3,000g for 10 minutes. Plasma samples were stored at -80°C. Fish were euthanized and both gonads were dissected for tissue samples. One gonad was fixed in neutral buffered formalin (NBF) for automated paraffin wax embedding and processing according to standard protocol.16 The second gonad was placed into separate cryomolds filled with OCT Compound™ (Tissue-Tek) and flash frozen in an isopentane/dry ice slurry at -80°C and preserved in the -80°C freezer until sectioned.17
Frozen tissue and paraffin wax sectioning
Frozen tissue blocks were sectioned at 5µm thickness using a Leica™ CM 1900 cryostat at a chamber temperature of -15°C and placed on #2 glass cover slips, coated with Gatenby’s Glue (27%v/v ethanol, 6.3%v/v acetic acid, 1.35% w/v gelatin, 0.09%w/v chrome alum, Gatenby & Beams.18 Glass cover slips were prepared in advance by dipping into Gatenby’s Glue solution and drying at room temperature in a bench top dryer with Drierite™ absorbent.19 Frozen sections were placed on the cover slips and set aside in Columbia jars to dry at room temperature. Sections were prepared for both hematoxylin and eosin (H&E) staining and Immunohistochemistry staining in duplicate; details are presented below. Paraffin wax blocks were sectioned at a 5µm thickness on a microtome and mounted onto slides using standard procedures.20
H&E staining, frozen and paraffin
Paraffin wax sections were deparaffinized and rehydrated according to standard protocols and stained with aqueous Harris H&E Y solution. The sections were again dehydrated and mounted using a xylene-based mounting medium. To stain frozen section cover slips with H&E, a modified version of the previous protocol were used E. Colley. Because the frozen sections were not fixed, dehydrated, or embedded with paraffin wax they did not need to go through deparaffinization, rehydration, or any antigen retrieval steps. The sections were compatible with aqueous stains eliminating the first few steps of the paraffin wax H&E protocol. Dried frozen sections on cover slips, were rinsed in room temperature distilled water (RTDW) to remove debris or dust, and placed in filtered Harris hematoxylin for 5 minutes. After a rinse in RTDW to remove excess stain, sections were quickly dipped into a 1% acid alcohol differentiator solution (1% Hcl and 70% alcohol), and immediately rinsed with RTDW to stop differentiation. Lithium carbonate 0.5% was used to blue the hematoxylin for 30 seconds, followed by a RTD wrinse to stop the bluing. Filtered 0.5% aqueous eosin Y was used as a counter stain for 30 seconds and then immediately washed in RTDW until all excess eosin was removed from the cover slips. The cover slips were then mounted onto glass microscope slides using an aqueous gel mounting media.
Immunohistochemistry staining
Immunofluorescence was performed using a modified standard immunofluorescence staining protocol for frozen sections.21 Sections were rinsed with 1x phosphate buffered saline (PBS) at pH 7.3, and then a blocking solution was added which was 2% bovine serum albumin (BSA) in PBS. Sections were incubated in the blocking solution for 30 minutes at room temperature to block the non-specific binding of antibodies then washed using 3 changes of PBS, 2 minutes each. The primary antibody was a polyclonal rabbit anti-sea bream VTG antibody (Biosense Laboratories™) prepared in a 1:1000 dilution in 1% BSA in PBS. Sections were incubated in the primary antibody for 1 hour at room temperature then washed again with 3 changes of PBS, 2 minutes each. The secondary antibody was a sheep anti-rabbit fluorescein isothiocyanate (FITC) antibody (Sigma-Aldrich™) specific for binding the rabbit primary antibody with a FITC fluorescent marker for detection. It was prepared in a 1:1000 dilution in 1% BSA in PBS. This step and all subsequent steps were performed in the dark. The sections were incubated in the secondary antibody for 30 minutes at 37°C and then washed with 3 changes of PBS for 2 minutes each. Sections were counter stained with Evan’s Blue (E2129, Sigma-Aldrich™) at a concentration of 0.002% for 1 minute. After washing with 3 changes of PBS for 2 minutes each, the sections were mounted with an anti-fade aqueous gel mounting medium and viewed under a Leica™ DM2000 fluorescence microscope.21
Microscope analysis
Sections from all fish samples were examined under the microscope for morphological characteristics, reproductive stage and to compare the quality of sectioning. H&E stained sections for both frozen and paraffin sections were examined using light microscopy and IF sections were examined using fluorescence microscopy. All microscope analyses were performed on a Leica Microsystems™ DM2000 fluorescence microscope equipped with a Leica™ DFC320 digital camera. IM500 software was used to take photos, to calibrate size bars and magnifications, and to measure individual cells for quantifying maturation. Key morphological components were labeled according to a salmonid gonad atlas.22,23 For each type of section, at least three photos were taken. Cell diameters of all cells in these three photos were measured using index vectors in the IM500 software. Cell diameters were compared to ranges listed for all six female reproductive stages.24 After cell counts were obtained for each stage and converted to percentages, they were averaged across the three photos for each female sample with standard deviation. For the IF sections, the presence of VTG was detected by evidence of strong fluorescence within the cells of the samples.
Blood plasma protein levels
Plasma samples were analyzed for total protein content using a standard Bradford protein determination method using bovine serum albumin as the standard.25 VTG content was determined using a previously described ELISA protocol using a 96 well flat bottom, half volume, and high binding polystyrene plate.8 The plate was incubated with rainbow trout VTG (Biosense Laboratories™, Bergen, Norway) as the standard, and the trout plasma samples. Similar to the IF protocol, the plate was blocked with a solution of 2% BSA (Sigma-Aldrich™). After blocking, the primary antibody was added, a rabbit anti-sea bream VTG polyclonal antibody PO-2 (Biosense Laboratories™). The secondary antibody was a goat anti-rabbit immunoglobulin G whole molecule peroxidase (Sigma-Aldrich™). The plate was read in a micro plate reader (Synergy™ HT, Bio-Tek Instruments, Winoosky, VT, USA) at a wavelength of 492nm, and the concentration of VTG in the plasma was found by comparison to the VTG standard calibration curve from 0–1,200ng/ml.26
Physiological indices
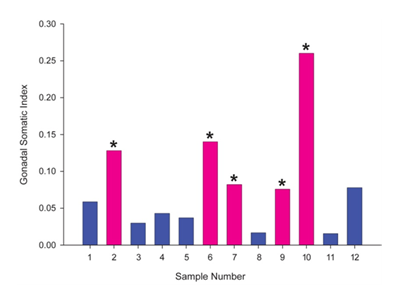
Mean condition factor, an indicator of the health of the fish was 1.25 with a standard error of the mean of 0.021. GSI, an indicator of the weight of the gonads as a percentage of the weight of the whole fish, demonstrated that the males had a significantly lower GSI than females (Figure 1).
Plasma VTG
Out of seven male specimens, three (samples no. 4, 11 and 12) had plasma VTG levels lower than the detection limit (25ng/ml) for this assay (Table 1). Males had a mean VTG concentration of 45.6(±63.6 SD) and females had a mean VTG of 61.0 (±43.8 SD)ng/ml (Table 1).
Sample |
Sex (M/F) |
Mean VTG concentration (ng/ml) |
SD |
1 |
M |
168 |
30.6 |
2 |
F |
25 |
72.1 |
3 |
M |
8.3 |
76.4 |
4 |
M |
ND |
NA |
5 |
M |
25 |
43.6 |
6 |
F |
55 |
85.4 |
7 |
F |
18.3 |
41.6 |
8 |
M |
81.7 |
130 |
9 |
F |
125 |
0 |
10 |
F |
81.7 |
15.3 |
11 |
M |
ND |
NA |
12 |
M |
ND |
NA |
Males |
45.6 |
63.6 |
|
Females |
61 |
43.8 |
Table 1 Plasma VTG Concentrations from ELISA
Staging gonad maturation
Analysis of both paraffin and frozen histological sections for gonad maturation in female samples yielded quantifiable cells in each stage of maturation (Figure 2A) (Figure 2B). In the frozen sections (A), samples 2, 6, 7 and 9 were dominated by stage I (20-200μm) and stage II (200-400μm) (nucleo cromatina and peri nucleolar) cells. These stages characterize the pre vitellogenic state when cells have a small diameter, cytoplasm stains uniformly pink with H&E stain, the nucleus takes up a large proportion of cell volume, there is a prominent nucleolus, and pre follicular cells can be seen surrounding the oocyte. At this stage, no yolk vacuoles or cortical alveoli were present. Sample10 had over 60% of its cells in stage IV or the medium oocyte stage (0.5-1mm, (Figure 2A). Stage IV is an indication that vitellogenesis is underway. The cell diameter is larger, yolk granules are prominent and start to connect into a flowing mass, nucleus becomes smaller and less prominent as it moves to the periphery, cortical alveoli merge their contents into the cytoplasm, and follicular cells for the complete corium. In the paraffin wax sections (B), over 50% of the cells in samples 2, 7, and 9 were in pre vitellogenic stages I and II, and samples 6 and 10 had a significant amount of vitellogenesis stage IV Medium cells.
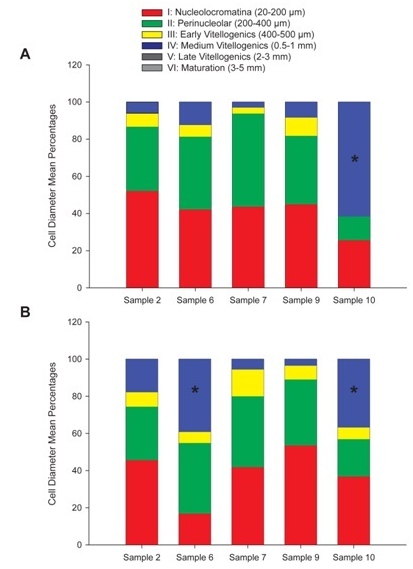
Figure 2 Stages of gonad maturation in female samples represented as mean cell diameter percentages for each stage of oocyte maturation I through VI.
Figure 2A Frozen sections, Sample 10 had a stage IV cell percentage significantly higher than that of samples 2,6,7 and 9. (T-test p-value <0.001, 0.05 significance level).
Figure 2B Paraffin wax sections, Samples 6 and 10 had significantly higher stage IV cell percentages than samples 2,7 and 9. (T- Test p-value <0.001, 0.05 significance level).
Comparative histology in H&E paraffin and frozen sections
H&E staining resulted in nuclei and membranes staining blue and the cytoplasm staining pink to red in both paraffin and frozen sections. Frozen sections of testis of sample 1 exhibited tightly packed follicles with small lumens (Figure 3A). The paraffin sections of sample 1 testis (Figure 3B) exhibited similar morphology; the follicles are tightly packed without much evidence of lumens. The nucleus stain was very prominent compared to surrounding structures. There was also evidence of small lobules in the surrounding gonadal tissue indicating an immature gonad. Frozen sections of testis sample 3 (Figure 3C), illustrated early lobule formation with the presence of small follicles with lumens where spermatocytes would develop and be released. Primordial germ cells, primary spermatocytes and macrophages along the follicle walls are an indicator of early spermatogenesis. The paraffin wax section (Figure 3D) exhibited very similar structures however it is evident that the follicles have more open lumens as well as space surrounding each follicle instead of the more crowded appearance of the frozen section, with the evidence of early lobule formation and some primary spermatocytes. Frozen sections from sample 6 ovary (Figure 3E) illustrated a developing female gonad with oocytes at multiple stages. There were small oocytes with well-defined nuclei and uniform cytoplasm; intermediate oocytes dotted with yolk vacuoles and cortical alveoli; and large oocytes with little cytoplasm, peripheral nuclei, and filled with yolk. The paraffin section of sample 6 (Figure 3F) exhibited a similar morphology with many immature cells scattered throughout larger cells. The cytoplasm stained prominently with cortical alveoli clearly distinguished from the nucleus. One main difference from the frozen section was the presence of membrane separation and splitting around the cells. Another was the presence of very densely staining small immature follicular cells. Frozen sections from sample 10 ovaries (Figure 3G) were more mature, containing large oocytes with hardly any stained cytoplasm and no nuclei. There were also very few immature cells surrounding the large oocytes. The cells are much larger in this gonad compared to the others, and the cells were pulling away from one another in the section. Also clearly illustrated was the tri-layered membrane of the larger oocytes, darkly staining in comparison to the surrounding structures. In the paraffin ovary section of the same stage IV (Medium) cells (Figure 3H), the cytoplasm held the stain better than the frozen section, but it was still pale. There was almost no evidence of nuclei with few intervening immature cells and few surrounding small cells. There was still evidence of membrane splitting and some distortion of oocyte shape.
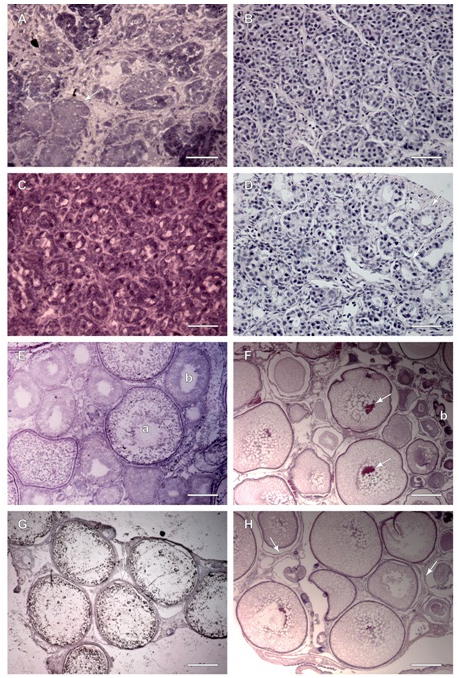
Figure 3 Paraffin and Frozen H&E stained sections, males (A, B, C and D) and females (E, F, G and H).
Figure 3A Frozen Section, sample 1 testis exhibits immature, tightly packed follicles (arrow) which will eventually form mature follicles with lumens and developing primary spermatocytes. Scale Bar is 50 µm.
Figure 3B Paraffin section, sample 1 testis exhibits immature tightly packed follicles throughout with better cellular detail visible. Scale Bar is 50 µm.
Figure 3C Frozen section, sample 3, testis follicles with lumens lined by primary spermatocytes and sertoli cells. Scale Bar is 50 µm.
Figure 3D Paraffin section, sample 3, testis follicles with better cellular detail, lumens are more evident and are lined by primary spermatocytes and sertoli cells (arrows). Scale Bar is 50 µm.
Figure 3E Frozen section, sample 6, ovary exhibits a large oocyte (a) filled with yolk without a prominent nucleus and very little stained cytoplasm, and a less mature oocyte (b) beginning to absorb yolk with vacuoles dotting cytoplasm. Scale Bar is 200 µm.
Figure 3F Paraffin section, sample 6, ovary exhibits maturing oocytes with dark staining nuclei (arrows) surrounded by many vacuoles (a). Immature cells (b) stain much darker. Scale Bar is 400 µm.
Figure 3G Frozen section, sample 10, ovary, majority of oocytes at stage IV: Medium, no nuclei, full of yolk, layered membranes seen (arrow), three layers surround the oocyte; zonaradiata, follicle and vascularized theca. Scale Bar is 400 µm.
Figure 3H Paraffin section, sample 10 ovary, with mature oocytes from stage IV: Medium, nuclei not evident in this section, many vacuoles spot cytoplasm, layered membranes are split apart (arrows), smaller oocytes surround larger ones, fewer cells seen due to oocyte reabsorption. Scale Bar is 400 µm.
Immunohistochemistry
Immunohistochemistry using frozen sections was performed on all samples. The presence of VTG was indicated by bright yellow florescence. The Evan’s Blue stained red the structures in the gonad that did not fluoresce. Male gonadal tissue did not exhibit a large amount of yellow fluorescence and the majority of the structures and cells stained with the counter stain (Figure 4A). The male gonads were filled with small nucleated cells which were congregated into bundles or immature lobules. All female gonadal tissue exhibited bright yellow fluorescence to varying degrees. Samples 2,6,7,9, and 10 (Figure 4B-4F) demonstrated that the yellow fluorescence was seen in the larger, more mature cells, but that the smaller cells with more pronounced nuclei took up more of the Evan’s Blue counter stain and stained a darker red color. Bright yellow fluorescence was depicted within the cells, but there were areas outside of the cell membranes in between cells with bright yellow fluorescence as well as illustrated in (Figure 4B,4C). Cortical alveoli are easily seen in photos of samples 6 and 7 (Figure 4C,4D). The VTG is seen streaming around the cortical alveoli and is visible as black dots inside the cells. Eventually cortical alveoli disappear as VTG dominates the interior of the cell, as can be seen in some of the larger cells. Nuclei are also clearly demarcated in both immature cells and the intermediate staged cells. Sample 9 (Figure 4E) illustrates a cell with a red staining nucleus surrounded by yellow fluorescent cytoplasm. The fluorescence was also useful for depicting the tri-layered membrane in some of the larger oocytes in sample 10 (Figure 4F). Although less consistent fluorescence is seen in the cytoplasm of these large cells, there are clumps of bright yellow fluorescence which indicates the presence of VTG.
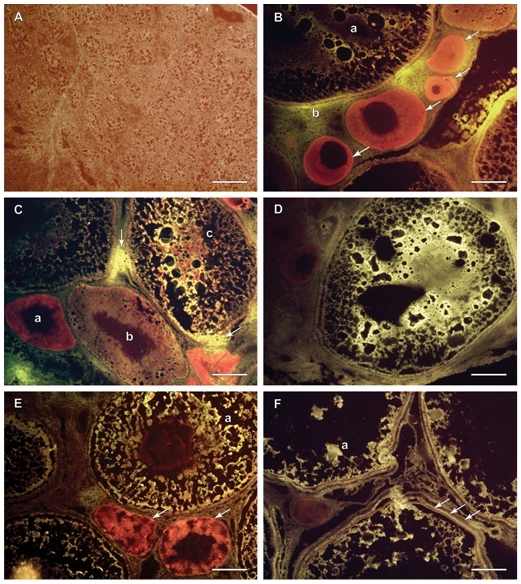
Figure 4 Frozen sections cut at 5 microns, IHC; males (A) and females (B, C, D, E and F).
Figure 4A Sample 1, testis, majority stained red with Evan’s Blue counter stain due to an abundance of nuclear material, membranes and cytoplasm, no bright yellow fluorescence present, therefore no VTG detected. Scale Bar is 100 µm.
Figure 4B Sample 2, ovary, immature cells stained heavily with red Evan’s Blue counter stain (arrows) indicating the absence of VTG, mature oocyte (a) with less red staining and more bright yellow fluorescence indicating VTG presence, some yellow fluorescence (b) is seen outside the membrane of the large oocyte indicating VTG presence in the interstitial space. Scale Bar is 100 µm.
Figure 4C Sample 6, ovary, immature small cell stains dark red (a), next largest cell stains lighter red with some yellow (b), the largest cell (c) has mostly bright yellow fluorescence indicating VTG presence as well as some amorphous red material where there are likely cellular components, some yellow fluorescence is seen in the interstitial space at two points (arrows) indicating VTG presence outside the cells. Scale Bar is 100 µm.
Figure 4D Sample 7, ovary, whole cell fluoresces very bright yellow indicating VTG absorption into the cell streaming around spaces where cytoplasm and yolk vacuoles are likely to be. There is no red nucleus present, either it was not represented in this section or it has been pushed to the side by the increasing cellular volume due to VTG absorption. There are dark red immature cells in the upper left corner of the photo. Scale Bar is 100 µm.
Figure 4E Sample 9, ovary, immature cells (arrows) and nuclei has darker red stain, a few spots in the largest oocyte (a) exhibit bright yellow fluorescence indicating VTG presence divided by black cytoplasmic areas, the nucleus stains dark red which will eventually become smaller and migrate to the periphery of the cell. Scale Bar is 100 µm.
Figure 4F Sample 10, ovary, oocyte membrane structure is distinguishable with 3 distinct layers (arrows), the zonaradiata, the follicle and the outermost layer is the vascularized theca. Some yellow fluorescent spots in these large stage IV Medium oocytes indicate the presence of VTG represented as a lesser percentage than in previous samples, mostly likely due to water absorption and VTG’s conversion to yolk product (a). Scale Bar is 100 µm.
Relating GSI, plasma VTG and gonad development
The fish used in this study exhibited a wide variation in their GSI’s. We presume this is mostly due to the mixed population of males and females and their young age. Females naturally have a higher GSI than males as they start to develop.27 The females in this study had a mean GSI that was three-fold higher than the males. Natural gonadal development in rainbow trout requires a total weight of approximately 600g and takes approximately 2-3 years for the gonads to fully mature.27 All of these fish were over 600g so the larger GSI in the females was not unexpected as they were starting gonadal development. One of the major outliers in regards to GSI was the female in sample 10 with an index of 0.26. This female had a VTG plasma concentration of 81.7µg/ml; this was not the highest VTG concentration seen. However, the cell size ratios illustrated that over 60% of the oocytes in the gonad were in the Medium stage (stage IV, cells 0.5-1mm in diameter). One might expect a fish with the largest GSI and exhibiting the most oocyte development to have the largest VTG concentration in the blood plasma, but this is not always the case. In the early stages of gonad maturation, steroid hormones work in a positive feedback mechanism to increase VTG concentrations in the blood. However, during the later maturation stages, steroids are regulated via negative feedback loops causing decreases in blood VTG.8 By comparison, sample 9 had the highest VTG plasma concentration in the female group, but had the lowest female GSI and the majority oocytes were in immature stages I and II. This is indicative of the beginning stages of natural gonad development. The positive feedback mechanism triggered by steroidal hormones from the ovary acts on the liver to produce VTG, which increases plasma VTG concentrations. However, there is a time delay between the induction of VTG production and physiological growth in the gonadal tissue as the oocytes absorb the protein.28
Fish 2, 6 and 7 all had similar average GSI’s with medium to low VTG concentrations in their blood plasma and the majority of oocytes in the immature stages. These other female fish could be classified as being sexually immature due to the lack of gonad maturity and low plasma VTG, but this does not mean that VTG was not present or that it was not being absorbed in the gonads in small amounts. Although reproductive stages have been defined, reproductive development is a continuous process and thus, VTG may be seen at low levels in females at immature stages of their life cycle.8 The male samples all had low GSI’s similar to the average GSI found in the controls for exposure experiments performed on juvenile rainbow trout.24 Plasma VTG concentrations, in male samples 4, 11 and 12 were below the lower detection limit of 25ng/ml previously reported in other studies.29 In the wild, there are external cues, such as temperature, photoperiod and season, which may cause a rapid increase in gonadal development.30 Living in a controlled, laboratory environment, these external cues are not present unless induced, thus the presence of immature gonads was expected in both males and females.
Comparison of sectioning methods
One important difference between paraffin and frozen sectioning is the simplicity of the frozen tissue preparation for sectioning and subsequent post-processing and staining. The fresh frozen method has very few steps and a shorter time to achieve the final product when compared to the paraffin wax method.13,16 Fresh frozen sectioning is used for rapid diagnostic processing which may be useful during experimentation to determine next steps.17 Morphological results can be obtained within minutes of sampling and IHC results can be achieved within hours with fresh frozen tissue. By comparison, it may require 3 days or more to obtain results with traditional histology. More equipment is required for paraffin wax histology, adding to the expense of the procedure. With fresh frozen sectioning, the only equipment needed is the -80°C freezer for storage, the cryostat for sectioning and the microscope for viewing. Commonly, paraffin wax or electron micrograph photos are more often published in journals as evidence. One explanation might be that, for some studies, especially those using high power microscopy, traditional paraffin wax histology can give a better resolution for some organelles. Good frozen sections rely on the interplay between specimen type, cryostat temperature, microtome knife temperature, and environmental temperatures and humidity.17 All of these must be optimal to cut consistently representative sections. When comparing tissue integrity, in frozen sections, the cell structure appeared to be more natural. Using traditional paraffin wax histology methods, the tissue undergoes extensive dehydration, paraffin embedding, deparaffinization, staining and then dehydration again for mounting. These steps cause shrinking and expansion of the tissue and can result in the shrinkage of cytoplasm away from membranes.11 This was seen in many of the paraffin sections in this study. However, the same amount of shrinkage was not evident in the frozen sections, indicating that frozen sectioning has the capacity to better preserve the integrity of the overall tissue structure compared to paraffin sectioning. Cell size ratios were calculated in this study for both paraffin and frozen sections. For the most part, there was no significant difference between the two methods. Fracturing within the nuclei of certain cells was also seen in paraffin sections. This can happen when rigid tissue such as cartilage or bone becomes brittle from being too dehydrated, or if there is chatter from the knife during sectioning.11 Adjustments can be made in these cases, such as softening rigid tissues with NaOH or EDTA. This same fracturing was not observed in the frozen sections, because the tissue was not subjected to the same amount of dehydration. In the frozen sections, membrane morphology was clearly observable as three distinct layers, with membranes stained as single dark lines. This is not observable in paraffin section photos at the same magnification. This could have been due to the fixation and dehydration steps in paraffin processing which caused cross linkages to form between structural proteins, resulting in uniform membranes instead of visible layers.11 Another observation was that nuclei stained darker red in the paraffin sections, especially in the immature cells, than in the frozen sections. Staining the frozen sections longer or changing the concentration of stain may alleviate this. Both paraffin and frozen tissue preparation and sectioning provided good depictions of morphology. Frozen sections exhibited less cellular damage than the paraffin sections, but the color contrast was better with paraffin. The paraffin protocol used for this study should be re-evaluated to attempt to reduce the amount of cellular damage.
Immunohistochemistry interpretation
The bright yellow fluorescence seen in the oocytes indicated the presence of VTG however the pattern of fluorescence was not the same in every sample. The largest cells in sample 10 exhibited dots of bright yellow florescence. There are a number of possible explanations for this. As oocytes mature, VTG is endocytosed into the cell and processed through cleavages by various cathepsin enzymes and stored in yolk vacuoles as the finished yolk product.31 Therefore, the VTG that existed while the cell was maturing was changed into yolk and the fluorescence may appear as a smaller percentage of the total volume assuming the cell is close to full maturation. Increased water absorption, which is common in the final stages of maturation, spreads out the other cellular components such as cytoplasm, VTG and yolk vacuoles making the fluorescence less homogeneous.31 Mature cells do not contain much VTG or cytoplasm relative to yolk protein and water and it is more difficult to see a consistent yellow fluorescence. The more immature the cell, the more cytoplasm in the oocyte.8 It was evident that in cells which are actively absorbing VTG, fluorescence was more uniform throughout the cell which was dotted by black vacuoles that would represent cellular components or yolk vacuoles.31 Although IF was not done with paraffin sections, the literature indicates that the paraffin method does not always allow for preservation of protein antigens in their natural functional state.12 This occurs via epitope destruction by paraffin processing, or the denaturing of proteins. The disruption of these intermolecular interactions can affect binding of the antibodies used for the immunofluorescence staining.12 The fixation and embedding can cause antigen masking. This ultimately results in antigen loss, and to overcome this problem, enzymatic or heat-mediated antigen retrieval is used. Antigen retrieval steps are not required on frozen sections because the sections have not been through the same fixation and embedding.11 For all these reasons, traditional paraffin sectioning is not the most ideal method for IF and thus, the method of frozen tissue sectioning is becoming routine for IF. The Immuno histo chemistry provided good examples of VTG detection. Not all cells expressed VTG presence, but this is expected in normal oocyte maturation. Not every oocyte will mature to become a late stage oocyte; many of the immature oocytes are reabsorbed.22 Immature and atretic oocytes as well as male gonadal tissue did not exhibit bright yellow fluorescence, but rather took up the counter stain. Counter stains such as Evan’s Blue have been used in other studies because it provides contrast to the bright fluorescence and masks background fluorescence.32 This was a positive result because it proved the IF method used was working and that the VTG antibodies did not bind other proteins. Using this hybrid of traditional histology methods and IF, researchers can directly detect the location of, and to some extent, the amount of, VTG in gonadal cells. Frozen sectioning produced excellent results with both H&E and IF. Paraffin histology remains the historically established method, which is ideal for finding organelles and producing clear photos with bright contrasting colors. Frozen sectioning maintains protein antigens, such as VTG, in their true form and is useful to demonstrate the presence of proteins of interest within tissues as a supplement to the molecular evidence of biomarker concentrations in the blood.
This study is preliminary and requires a more in depth investigation with a larger number of fish and representative tissue samples and the inclusion of different species. It would be useful to perform serial sections for frozen and paraffin histology and perform IF on both to compare the two methods for VTG detection. Utilizing frozen sectioning and IF with other tissues, such as the liver, for the detection of VTG would indicate how the VTG pathway is functioning and provide a baseline for comparison with changes due to reproductive endocrine disruption. Frozen sectioning and IF for VTG would be useful for tissue analysis in exposure experiments to possible EDCs. Also, using IF to look for other biomarkers such as enzyme complexes or steroid hormones would provide another dimension to the analysis. Optimizing the protocols for use with different species of fish as part of multi-trophic studies to compare morphology and effects would expand the applicability of these methods.
The authors wish to thank Elizabeth Colley for her expertise in histology and Dr. Rodrigo Orrego for his assistance, guidance and expertise. The authors are grateful for the constructive comments of the anonymous referees which significantly improved the manuscript.
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: This work was supported by grants from Tier 1 Canada Research Chair in Aquatic Toxicology (CRC Grant # 950-221924); Natural Science and Engineering Research Council of Canada (NSERC Grant # 360557-2009).
The author declares no conflict of interest.

©2015 Krause, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.