MOJ
eISSN: 2379-6294


Research Article Volume 1 Issue 3
1Department of Environmental Toxicology, National Institute of Pathology (ICMR), India
2Centre for Animal Disease Research and Diagnosis (CADRAD), Indian Veterinary Research Institute, India
Correspondence: Arun Kumar Jain, Department of Environmental Toxicology, National Institute of Pathology (ICMR), Safdarjung Hospital Campus, New Delhi-110029, India, Tel +91 11 26198402, Fax +91 11 26198401
Received: May 15, 2014 | Published: July 29, 2015
Citation: Kumar SN, Telang AG, Singh K, et al. Toxic manifestation of endosulfan and ochratoxin- a in adult male rats. MOJ Toxicol. 2015;1(3):96–101. DOI: 10.15406/mojt.2015.01.00012
The study examined effects of Ochratoxin-A (OTA) and Endosulfan on the haemato-biochemical changes in rats. Adult male Wistar rats were randomly divided into four groups and fed OTA @ 4ppm in feed (Group I); endosulfan @ 5mg/kg body weight in corn oil by oral intubation (Group II); combination of both endosulfan @ 5mg/kg body weight in corn oil and OTA @ 4ppm in feed (Group III) and toxin free feed (Group IV – control group) daily for 30 days. After treatment, blood was collected from heart for studying haemato-biochemical indices. All toxin treated rats showed a significant (P<0.05) decrease in the body weights. The treated rats became anemic (normocytic hypochromic anemia) and also revealed lympho cytopenic-leukopenia. Biochemical changes included significant decline in, serum globulin, total protein and albumin along with concurrent increased levels of blood glucose, AST and ALT. Spleen of the treated animals showed depletion of lymphocytes on light microscopy. The present investigation revealed significant toxicity in combination group in comparison to single exposure to OTA or endosulfan alone (Group I and Group II, respectively). These findings suggest that concurrent exposure to endosulfan and OTA results in additive toxic manifestation on haemato-biochemical parameters in male rats.
Keywords: ochratoxin-a (ota), endosulfan, normocytic normochromic anemia, lymphocytopenia, leucopenia
OTA, ochratoxin-a; HR, hour; PPM, parts-per million; B.Wt., body weight; RBC, red blood cell; Hb, hemoglobin; PCV, packed cell volume; TLC, total leukocyte count; DLC, differential leukocyte count; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; BUN, blood urea nitrogen; ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase
Contamination of agricultural produce by pesticides and/or mycotoxins can result in severe problems both for human health as well as the economic value of crops. Mycotoxins are fungal metabolites produced by several molds that contaminate various agricultural commodities during crop production, or in storage, where drying technology may be preventative. Ochratoxin-A (OTA), produced by several species of genera Aspergillus and Penicillium is a common contaminant of different food or feedstuffs which can cause toxicities in human beings and animals.1 OTA can result in several deleterious health effects causing – neurotoxicity,2 nephrotoxicity,3 genotoxicity,4 immunotoxicity,5 carcinogenicity6 and teratogenicity7,8 in various mammalian species. Occurrence of OTA has also been reported in humans’ serum, breast milk and kidneys suggesting its public health significance.9
Pesticides are used to protect crops from damage caused by pests including molds and pathogens. Exposure of the general population to pesticides can occur by direct application to food commodities (to increase their shelf-life) and/or through drinking water contaminated with pesticide residues.10 At low level many pesticides have potential toxic effects on non-target organisms and may interfere with the endocrine system.11 Endosulfan is one of the most commonly used organochlorine pesticides, which is lipophilic in nature. Because of its persistence in the environment, its usage has been banned in most developed countries. However, it is still being used in the developing countries because of its availability through illegal importation.12 The perused literature showed some information on the contamination OTA and endosulfan in adult male rats,4,13 but reports on their toxic effects in animals are limited. Moreover, no report could be traced in the literature on the combined effect of OTA and endosulfan in male rats, although both may occur as co-contaminants under field conditions in certain areas. It is with this view that this study was carried out to investigate the combined toxic effects of endosulfan and OTA on the hematological and biochemical indices of male rats.
Production and analysis of Ochratoxin-A
A pure culture of Aspergillus ochraceus NRRL-3174 originally procured from National Centre for Agriculture Research (NCAUR-3174) Peoria, Illinois, USA was grown on sterilized maize as per the method described by Trenk et al.14 The extraction and clean up of the toxin sample was done as per the method of AOAC.15 Cultured maize powder containing known amount of OTA was added to basal ration in such a proportion that the final concentration of OTA was adjusted to 4 ppm level in the feed.13
Animals and experimental design:
Male Wistar rats (n=40) with average weight 160±10g were procured from Laboratory Animal Resource Section of Indian Veterinary Research Institute (IVRI), Izatnagar, Bareilly, Uttar Pradesh, India. Animals were housed in polypropylene cages in an artificially illuminated room (12-hr light: 12-hr dark cycle) free from any source of chemical contamination. The temperature and relative humidity of the room were maintained at 22±3°C and about 50-60%, respectively. Rice bran was used as bedding material which was changed on every alternate day. The rats were provided with standard laboratory animal feed and water ad libitum. After fifteen days of acclimatization period, they were randomly assigned into four groups viz. Group-I, served as a negative control and rats received standard toxin-free feed; Group-II, rats received diet containing OTA alone at a dietary level of 4ppm (as explained above); Group-III, rats received endosulfan alone dissolved in corn oil at a concentration of 5mg kg-1 b.wt. by oral intubation daily; and Group-IV, rats received both OTA (4ppm) and endosulfan (5mg kg-1 b.wt.) throughout the experiment.13 The experiment on rats was approved by the Animal Ethical Committee of IVRI, Bareilly. The dose selection criteria for the present stud decided on the basis of oral median lethal dose (LD50) of OTA (20-30mg kg-1 b.wt.) and endosulfan (80mg kg-1 b.wt.) in rats. Considering a rat weighing 160g, daily consumes about 25g diet containing 4µg OTA/g diet, approximately, 1/30th oral lethal dose (LD50) levels for OTA (4ppm) and 1/15th oral lethal (LD50) dose levels for endosulfan (5mg kg-1 b.wt.) were selected and used in the present investigation.
Hematology
At the end of the study i.e. after 30 days post-intoxication, the blood samples were collected by cardiac puncture into heparinised vials during the sacrifice of animals. Complete hemogram, including red blood cell count (RBC), hemoglobin (Hb), packed cell volume (PCV), total leukocyte count (TLC) , differential leukocyte count (DLC), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) were estimated by using standard reference methods of Jain.16
Serum biochemistry
Total serum protein, albumin (modified Biuret & BCG dye binding methods), blood urea nitrogen (BUN) (DAM method), serum creatinine, serum alkaline phosphatase (ALP), serum alanine transaminase (ALT), serum aspartate transaminase (AST), serum calcium and phosphorus were estimated by Commercial Biochemical kits (Span Diagnostics Ltd. Gujarat, India). The values of globulin were derived by subtracting the values of albumin from that of total protein.
Pathological changes
Spleen from rats of all the groups was examined for gross changes. Representative tissue samples were collected in 10% buffered formalin for histopathological studies. The tissue samples were properly processed and embedded in paraffin wax. The sections were stained with routine hematoxylin and eosin stain17 and were then examined under light microscope.
Data generated during the study were suitably analyzed using one way Analysis of Variance (ANOVA) to detect differences among groups and the means were compared by Dunnett’s multiple comparison test. All analyses were performed with Graph Pad In Stat software (San Diago, USA). All the statements of significance were based on a probability level of p<0.01.
There were no noticeable clinical signs and symptoms. During the last two weeks of the experiment, rats from toxin fed group appeared dull, depressed and anorexic. The rats of control group remained active and alert throughout the experiment. Weekly body weight of the rats receiving OTA, endosulfan and their combination for 30 days were recorded. There was significant reduction (P<0.05) in the body weight of rats given combination of OTA and endosulfan from 1st week onwards and from 2nd week onwards in the rats given OTA alone as compared with control group (Table 1). There was a non-significant reduction in weekly body weight of the rats belonging to endosulfan treated group (Table 1). Effect of 30 days treatment with OTA, endosulfan and their combination on various hematological parameters was studied (Table 2). There was significant reduction in the mean hemoglobin (Hb) values of the rats administered OTA, Endosulfan and their combination than those of control. There was marked reduction in Hb values in combination group as compared to exposure to OTA or endosulfan alone. The PCV values were significantly reduced in rats receiving combination of two toxins as against in control rats. However, there was no reduction in the PCV values when OTA and endosulfan were administered individually to the rats. The values of TLC were significantly lowered in rats receiving OTA, endosulfan and their combination as compared to untreated rats. However, there was no significant difference amongst the three treatment groups. The RBC values remain unaltered due to OTA and Endosulfan treatment from that of untreated control. However, the combination treatment caused significant reduction in the RBC count as compared to remaining three groups. Rats of combination group i.e. (OTA+Endosulfan) showed significant decrease in derived values of MCV, MCHC and MCH. On the other hand, in comparison to control, significantly lower values of MCHC and MCH were observed in the OTA treated group. Blood leukocytes revealed fragmentation and formation of round globular structures in the cytoplasm indicating apoptosis in all the three experimental groups. Differential Leukocyte Count did not show much variation except neutrophilia (56%) in OTA group with corresponding lymphocytopenia (36.7%). The values of several biochemical indices were found to be decreased in all the three treatment groups as compared to control (Table 3). The reduction was registered in levels of serum total protein, albumin, globulin, and calcium and phosphorous. On the contrary levels of serum glucose, ALP, creatinine, ALT, AST and BUN were found to be elevated in the three treatment groups. The variations i.e. increase as well as decreases were more pronounced the combined treatment group IV of OTA+Endosulfan (Table 3).
Group → |
Control |
OTA |
Endosulfan (ES) |
Combination (OTA + ES) |
Significance |
Weeks ↓ |
Body Weight (gm) Expressed as Mean± SE (n=10) |
||||
0 |
155.0±8.42 |
156.67±7.26 |
156.67±6.67 |
158.25±3.12 |
Non significant |
1st |
157.5±7.77b |
150.0±7.64 |
156.0±3.05 |
137.5±3.23a |
P≤0.05 |
2nd |
160.25±7.19b |
138.67±5.20a |
155.33±2.67b |
131.5±3.01a |
P≤0.05 |
3rd |
165.00±6.45b |
134.33±5.36a |
151.67±3.84b |
122.5±3.23a |
P≤0.05 |
4th |
171.00±7.08c |
128.67±4.18ab |
148.33±5.67bc |
112.5±2.50a |
P≤0.01 |
Table 1 Effect of OTA (4 ppm) and Endosulfan (5 mg kg-1 b.wt) administered alone and in-combination on weekly body weight of adult male rats
Mean bearing at least one common superscript do not differ significantly between groups
Parameters |
Control |
OTA |
Endosulfan |
Combination (OTA + ES) |
Significance |
Hemoglobin (g/dl) |
13.78±0.175d |
9.22±0.35b |
12.15±0.48c |
7.86±0.31a |
P≤0.01 |
PCV (%) |
40.52±0.83a |
37.18±0.95a |
38.56±1.32a |
31.36±1.73b |
P≤0.01 |
TLC (X103/μl) |
12.74±44b |
10.42±0.54ab |
11.51±0.50a |
10.13±1.11a |
P≤0.05 |
RBC (106/μl) |
6.62±0.155b |
6.12±0.172b |
6.43±0.22b |
5.29±0.29a |
P≤0.01 |
MCV (fL) |
60.94±0.60b |
60.73±0.48b |
60.76±0.36b |
58.19±1.27a |
P≤0.05 |
MCHC (%) |
34.31±0.77c |
23.58±0.90a |
31.86±1.77bc |
27.10±1.85ab |
P≤0.01 |
MCH (pg) |
20.80±0.44b |
14.28±0.57b |
19.11±0.98b |
14.73±1.27a |
P≤0.01 |
Neutrophil (%) |
31.30±0.70a |
56.0±1.01b |
29.5±0.909a |
30.60±0.83a |
P≤0.01 |
Lymphocyte (%) |
65.30±0.47a |
36.70±0.87a |
65.70±0.67a |
64.9±0.90a |
P≤0.01 |
Monocyte (%) |
1.60±0.27a |
5.00±0.47c |
2.40±0.16ab |
3.70±0.47bc |
P≤0.01 |
Eosinophil (%) |
1.00±0.42a |
2.00±0.26ab |
2.4±0.37b |
1.0±0.25a |
P≤0.01 |
Basophils (%) |
0.80±0.25a |
0.0b |
0.0b |
0.0b |
P≤0.01 |
Table 2 Effect of OTA and Endosulfan (ES) administered alone and in combination on hematological parameters (Values expressed as Mean ± SE; N=10) in adult male rats
Mean bearing at least one common superscript do not differ significantly between groups. ES= Endosulfan
Parameters |
Control |
OTA |
Endosulfan |
Combination (OTA + ES) |
Significance |
Glucose (mg/dl) |
80.13±2.10a |
100.23±2.38b |
100.38±1.58b |
115.07±1.89c |
P≤0.01 |
Total protein (g/dl) |
7.82±0.204b |
6.58±0.365ab |
7.22±0.37ab |
6.29±0.34a |
P≤0.01 |
Albumin (g/dl) |
4.34±0.153b |
3.62±0.233ab |
4.05±0.223b |
3.08±0.244a |
P≤0.01 |
Globulin (g/dl) |
3.59±0.280b |
2.603±0.29a |
2.55±0.337a |
2.66±0.268a |
P≤0.05 |
AG ratio |
1.84±0.075 |
1.86±0.107 |
1.78±0.17 |
1.95±0.21 |
Non significant |
ALP (IU/L) |
84.6±0.194a |
106.7±0.62b |
104.7±0.726b |
117.0±0.92b |
P≤0.05 |
Creatinine (mg/dl) |
1.07±0.060a |
1.87±0.193bc |
1.51±0.18ab |
2.26±0.227c |
P≤0.01 |
Calcium (mg/dl) |
9.36±0.23b |
8.84±0.23ab |
8.97±0.23ab |
8.33±0.17a |
P≤0.01 |
Phosphorus (mg/dl) |
8.49±0.224b |
8.07±0.24ab |
8.00±0.21ab |
7.59±0.196a |
P≤0.01 |
BUN (mg/dl) |
19.52±0.52a |
24.01±0.68bc |
21.99±0.567ab |
26.00±0.92c |
P≤0.01 |
ALT (U/L) |
79.17±3.082a |
103.81±2.58b |
94.32±3.041b |
133.16±2.93c |
P≤0.01 |
AST (U/L) |
87.68±1.74a |
96.27±3.89a |
103.18±3.76a |
144.45±9.28b |
P≤0.01 |
Table 3 Effect of OTA and Endosulfan (ES) administered alone and in combination on biochemical parameters in adult male rats
Values expressed as Mean ± SE (N=10)
Mean bearing at least one common superscript do not differ significantly between groups ES= Endosulfan.
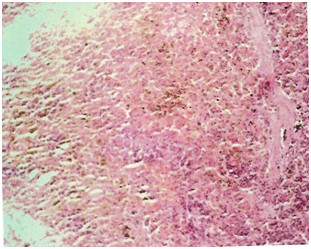


While samples of spleen from control animals showed the presence of parenchyma with mild hemosiderin pigment on light microscopy, sections from OTA and endosulfan treated animals showed focal depletion of lymphocytes in some of the peri-arteriolar lymphoid tissue and hemosiderin deposit in parenchyma (Figure 1 & 2). Animals exposed to Endosulfan and OTA combination had splenic corpuscles with poor population of lymphoid cells along with presence of hemosiderin pigment (Figure 3).
In this study, the rats of the control group as well as all the three experimental groups weighed approximately 150 to 160gm on day zero. As expected the control rats gained weight during 4-weeks experiment and their weight increased to 171±7gm. In the treatment groups, however, all the rats showed variable decline in the weight during the course of experiment and the lowest body weight was recorded in combination group treated with endosulfan and OTA. Our finding on reduction weight are consistent are with previous reports.18,19 The study further revealed OTA-induced normocytic hypochromic anemia. There was also reduction in total leukocyte count marked by lymphocytopenia along with increase in neutrophil percentage. The literature available regarding the effects of OTA on hematological parameters is contradictory and inconsistent.20,21 Mild leukocytopenia and lymphocytopenia observed in the present study could be associated with lymphoid cell depletion from lymphoid organs as observed during histopathological examination of spleen. OTA-induced leukocytopenia associated with lymphocytopenia has been reported previously in several experimental animals including rats.22 Endosulfan caused the lymphocytopenic leukocytopenia, which were reported in mice.23 Reduction in hemoglobin and PCV values, suggestive of anemia in the present study are in accordance with earlier reports in poultry,24 mice23 and pregnant rat.25 Anemia might be attributed to low nutrition intake, reduced protein synthesis and increased protein excretion in urine. Leukocytopenia was essentially lymphocytopenia type which might be correlated with decreased number of lymphocytes in spleen and Peyer’s patches seen histopathologically. Similar changes of mild depletion of lymphocytes in spleen have also been reported.26 The anemia was more pronounced in OTA and endosulfan combination group probably due to their synergistic effects on heamatopoietic system.
Biochemical alterations in OTA treated rats were decrease in serum total protein, albumin, globulin, calcium and phosphorus levels. Decreased serum total protein, albumin, globulin values might be due to OTA-induced damage to liver,27 the major site of protein synthesis.28,29 OTA has been shown to inhibit the protein synthesis. Further, proteinuria especially albuminuria resulting from toxic damage to nephrons could have resulted in decreased albumin fraction observed in the study and corresponding reduction of A:G ratio.30 Decrease in calcium and phosphorus levels is in agreement with reported decline in OTA-treated Broiler chicken.31,32 Along with reduction in protein, calcium and phosphorus, OTA toxicity resulted in significant increase in the blood glucose levels. This finding was akin to the report of the earlier workers in rats33 and pig29 who recorded increase in blood glucose level in ochratoxicosis. It is possible that the depletion of glycogen from liver might have resulted in increased blood glucose levels.34 Hyperglycemia in ochratoxicosis may be due to the inhibited synthesis and/or reduced release of insulin from the pancreatic cell.33
Rats receiving endosulfan by oral intubation also showed decrease in serum total protein, albumin, globulin, calcium and phosphorous and the increase in levels of glucose, ALP, ALT, AST and BUN which is in accordance with the previous publications.31 Decrease in total protein values might be due to low feed intake, inadequate digestion and/or absorption due to endosulfan induced damage in gastro-intestinal tract,13 liver damage, anorexia and hepatotoxic activity of endosulfan.35 As in the case of OTA-induced toxicity, severe damage to nephrons could result in proteinuria especially albuminuria thereby causing decrease in serum albumin fraction and corresponding reduction in A:G ratio. Decrease in levels of calcium and phosphorous has also been reported earlier.36 Increased blood urea nitrogen (BUN) may be due to prerenal causes (cardiac decompensation, water depletion due to decreased intake, and increased protein catabolism) or renal causes (tubular necrosis) and is in agreement with earlier reports in rats.35,37 Significantly increased blood glucose level in OTA and endosulfan treated animals is in agreement with the previous findings of increased blood glucose level after oral endosulfan and dimethoate administration38,39 and could be due to decreased level of insulin synthesis from pancreatic cells, increase in glucogenesis and glycogenolysis as well as inhibition of glucogenolysis and glycogenesis during stress.40
It can be deduced from this study that individual as well as combined exposure of OTA and endosulfan has the potential to damage the structural integrity and physiological activities of the organism which led to changes in haemato-biochemical parameters. Further, concomitant exposure to more than one toxin may result in additive toxic manifestations.
None.
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.

©2015 Kumar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.