MOJ
eISSN: 2379-6294


Research Article Volume 5 Issue 2
Department of Biochemistry, Lagos State University, Nigeria
Correspondence: Olabisi O Ogunrinola, Tissue Culture Research Laboratory (Drug Discovery Unit) Department of Biochemistry, Lagos State University, Ojo, PMB 0001, LASU Post office, Lagos Badagry Expressway. Lagos – Nigeria, Tel +234 (0) 803324476
Received: March 26, 2019 | Published: April 17, 2019
Citation: Ogunrinola OO, Fajana OO Adu OB, et al. The effects of Vernonia amygdalina leaves on lipid profile in cadmium-induced rat. MOJ Toxicol. 2019;5(2):83-87. DOI: 10.15406/moji.2019.05.00159
Medicinal plant, Vernomia amygdalina (Va) have been used in folk medicine in the treatment of several illnesses and diseases, its effect on lipid metabolism in metal induce toxicity remains enigmatic. In order to investigate the effects of Va on induced-cadmium, sixty four male albino rats were randomly divided into four major groups (n=16). Group 1 (A and B) served as control for both cadmium and Va exposed groups, while the remaining groups (2 - 4) were exposed to 100, 200 and 300 ppm cadmium as cadmium chloride in their drinking water for 6 weeks. At the end of 6 weeks, all rats in group IA, IB, IIA, IIIA and IVA (n=8) in were sacrificed. The remaining rats in groups II, III and IV (n=8) were given Va for seven days, then sacrificed. Blood was collected and organs (liver and brain) were excise for lipid profile (cholesterol, triglyceride and phospholipid) analyses using a spectrophotometric method. The hallmark of cadmium induction is dyslipidemia. A significant (p<0.05) hypolipidemia was observed in the Va treated animal at all doses. A similar observation was shown in erythrocyte triglyceride and phospholipid. There is significant (p<0.05) dose-dependent up/down regulation of brain cholesterol, triglyceride and phospholipid concentration with the administration of Va. While the intake of Va decreased hepatic cholesterol and triglyceride there is an increase in phospholipid concentration. The results show that cadmium-induced and treatment with Va leaves has up and down regulatory effect on the lipid profile of male albino rats.
Keywords: Vernomia amygdalina, cadmium, hypolipidemia, erythrocyte, Lipid profile, brain
Medicinal plants from ancient time, have been used for preventive and curative measures for different ailments and diseases due to their readily availability and low cost of preparation.1 Large population of humans still rely on plants as a source of medicine,2,3 therefore, the World Health Organization,4 recommended further investigation into medicinal plant, particularly in the area of chronic and debilitating illness and diseases such as infertility, diabetes, malaria, high blood pressure, dysentery, worm infestation, cancer, diarrhea, cardiovascular disease and many more illness/diseases. The medicinal plant, Vernonia amygdalina (Va), popularly called bitter leaves belong to the family Asteraceae or Compositae, is consumed locally as food and for ethno-medicinal uses. Va bitter taste is derive from the anti-nutritional components of the leaves, such as alkaloids, saponins, glycosides, tannins.5−8 As well as the flavonoids, oxalates, phytates,8 terpenes, steroids, coumarins, phenolic acids, lignans, xanthones, anthraquinones, essential oil and sesquiterpenes,9,10 that attributed to its pharmacological functions,11 as anti-diabetic, antimalarial, anti-helminth, antibiotic,12 treatment of diarrhoea, dysentery, fertility inducer, kidney problems, stomach discomfort,13,14 and hypolipidaemic, among other several uses. The dyslipidemia of heavy metal-cadmium (Cd) induction have been reported by Ogunrinola15 in rat model. Cadmium, an environmental contaminant from smoking, air pollution, occupational exposure such as battery industry and fertilizers, gets to the human body though foods, water and undergo bioaccumulation endangering human health.16−18 Lipids molecules are fatty acid, cholesterol, triglycerides and phospholipids that are play key roles in metabolism of living organism.19 This research work was carried out to investigate the effects of dried leaves of Va lipid profile of cadmium induced rat.
Collection and preparation of plant material
The leaves of Vernonia amygdalina (Va) plant was freshly collected from Ojo community market (Iyana Iba) in Lagos State. The leaves was authenticated by the Botany Department, Lagos State University, Ojo, Lagos. They were cleaned, air-dried and stored for future use.
Animals grouping and treatment
Sixty four male albino rats weighing between 100 - 200 g bred in the Animal House of the Department of Biochemistry; Faculty of Sciences; Lagos State University, Ojo-Lagos, Nigeria was used for the study. The animals were housed in stainless cages to acclimatize for two weeks under 12h light/dark cycle. They were allowed water and food freely. The animals were divided into four (4) groups (n=16) based on the research of Yapping et al.20
Group IA: Normal feed (grower mash), distilled water for 6 weeks (control)
Group IB: 10 g dried Va leaves + normal feed (grower mash), distilled water (Va leaves control)
Group II: Normal feed (grower mash), cadmium chloride in drinking water (100ppm) for 6 weeks
Group IIB: Normal feed (grower mash), cadmium chloride in drinking water (100ppm) for 6 weeks and 10 g dried Va leaves + Normal feed for 7 days.
Group III: Normal feed (grower mash), cadmium chloride in drinking water (200ppm) for 6 weeks
Group IIIB: Normal feed (grower mash), cadmium chloride in drinking water (200ppm) for 6 weeks and 10g dried Va leaves + Normal feed for 7 days.
Group IV: Normal feed (grower mash), cadmium chloride in drinking water (300ppm) for 6 weeks
Group IVB: Normal feed (grower mash), cadmium chloride in drinking water (300ppm) for 6 weeks and 10g dried Va leaves + Normal feed for 7 days.
At the end of 6 weeks and 7 days of treatment, animals were fasted overnight, and sacrificed under light ketamine anaesthesia. Blood was collected from the animals into heparinised tubes by cardiac puncture and separated into plasma and erythrocyte. The brain and liver were removed from the animals, homogenized and supernatant stored. All samples were analysed for lipid profile (cholesterol, triglyceride and phospholipid). All experiments were performed in compliance with the Ethical guide for the care and use of laboratory animals.21
Plasma lipid profiles
Determination of the major plasma lipids (cholesterol, triglyceride, and phospholipid) followed established procedures. Details of these have been given in our earlier researches.15,22,23
Organ and erythrocyte lipid profiles
The liver and brain lipids were extracted as described by Folch et al.24 and erythrocyte lipids followed the method described by Rose and Oklander25. After washing with 0.05 M potassium chloride (British Drug House) solution, aliquots of the lipid extracts were then used for the determination of lipid profiles spectrophotometrically by details described earlier.15,22,23
Statistical analysis
Results are expressed as mean±S.E.M. One-way analysis of variance (ANOVA) followed by Turkey’s test (Turkey honest significant difference (THSD)) was used to analyse the results with p<0.05 considered significant.
As indicated in Figure 1, the induction of cadmium elicited significant down regulation of plasma cholesterol (A), triglyceride (B) and phospholipid (C) concentrations at all doses and treatment with dried Va leaves further decreased the lipid contents at all doses. Likewise in Figure 2, cadmium induction resulted in significant (p< 0.05) decreased of erythrocyte lipid profile concentrations at all doses and treatment with Va significantly increased in triglyceride concentration at 100 ppm dose (139.11±22.69 mg/dl), decreased in control and 200 ppm dose and no significant difference in 300 ppm dose. Also, treatment with Va reduced phospholipid concentration in the control and 100 ppm dose but significantly increased at 200 and 300 ppm doses.
Figure 3 depicts the effect of Va on cadmium-induced brain lipid contents of the animal. Induction with cadmium resulted in the down regulation of brain cholesterol, triglyceride and increased phospholipid concentrations. Treatment with dried Va leaves caused dysregulation in cholesterol, up-regulation in triglyceride and phospholipid concentrations at all doses respectively. The hepatic lipids contents of the animals are shown in Figure 3. Cadmium induction and treatment with dried Va leaves reduced hepatic cholesterol and triglyceride concentrations and increased phospholipid concentration at all doses.
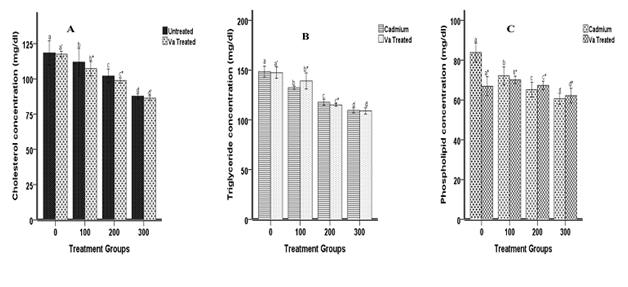
Figure 1 Effects of cadmium and Va on erythrocyte cholesterol (A), triglyceride (B) and phospholipid (C) concentrations. Each bar represents the mean±S.E.M of 8 rats. Bars with different alphabets are significantly different at p<0.05.
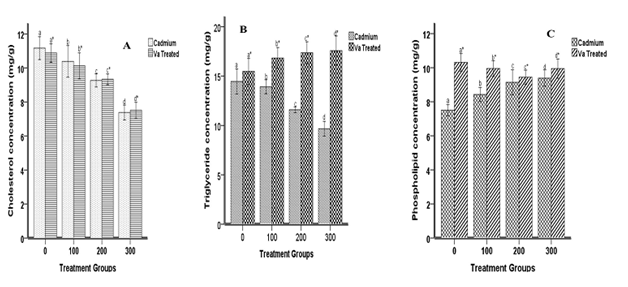
Figure 2 Effects of cadmium and Va on brain cholesterol (A), triglyceride (B) and phospholipid (C) concentrations. Each bar represents the mean±S.E.M of 8 rats. Bars with different alphabets are significantly different at p<0.05.
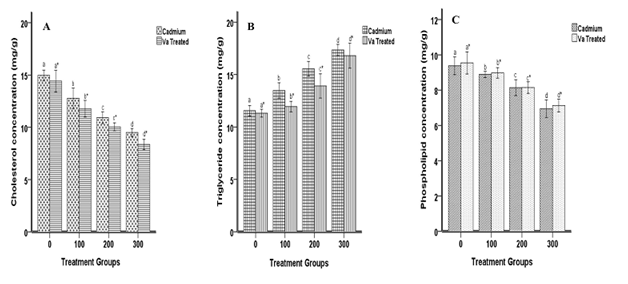
Figure 3 Effects of cadmium and Va on liver cholesterol (A), triglyceride (B) and phospholipid (C) concentrations. Each bar represents the mean±S.E.M of 8 rats. Bars with different alphabets are significantly different at p<0.05.
The ratios of cholesterol to phospholipid in the plasma, erythrocyte, brain and liver of rats as a result of cadmium–induction and treatment with Va are depicted in Table 1. Plasma cholesterol resulted in decreased ratio in the control group (1.63±0.11), increased ratio in the 100 ppm (2.96±1.52) and no significant difference in the ratios of 200 and 300 ppm respectively. With the exception of the control group in erythrocyte, the Va treatment resulted in decreased ratios from 100 ppm (1.54±0.09) to highest dose of cadmium-induced (1.36±0.08). The brain ratio increased in the control, 200 ppm groups and reduced at 100 and 300 ppm doses of cadmium-induced. The liver ratios increased in all the groups except 100 ppm that decreased with Va treatment.
Cadmium Dose |
Treatment Groups |
Plasma |
Erythrocyte |
Brain |
Liver |
0 |
Cadmium-induced |
1.69±0.20a |
1.46±0.15a |
1.33±0.10a |
1.37±0.10a |
Va-Treated |
1.63±0.11a* |
1.81±0.11a* |
1.40±0.19a* |
1.57±0.16a* |
|
100 |
Cadmium-induced |
1.59±0.10b |
1.60±0.18b |
1.23±0.08b |
1.23±0.10b |
Va-Treated |
2.96±1.52b* |
1.54±0.09b |
1.07±0.14b** |
1.21±0.11b |
|
200 |
Cadmium-induced |
1.21±0.03c |
1.59±0.11c |
1.40±0.12c |
1.29±0.14c |
Va-Treated |
1.21±0.04c |
1.48±0.06c* |
1.59±0.14c |
1.68±0.16c |
|
300 |
Cadmium-induced |
1.01±0.07d |
1.43±0.05d |
1.27±0.12d |
0.95±0.05d |
Va-Treated |
1.00±0.09d |
1.36±0.08d* |
1.18±0.17d |
1.21±0.08d* |
Table 1 Ratio of cholesterol to phospholipid in plasma, erythrocyte, brain and liver of control of the experimental animals
Lipid irregularities play a significant role in the pathogenesis and progression of atherosclerosis and cardiovascular disease and reports that environmental factors contribute to these conditions.26,27 The use of medicinal plants for therapeutic purposes are increasingly becoming prevalent in modern society as alternatives to synthetic medicines. This study provides experimental evidence of dried Vernomia amygdalina (Va) leaves on cadmium-induced lipid profile in male albino rat through drinking water. The hallmark of cadmium-induced is dyslipidemia, as observed in this study and has been reported by earlier studies.15,22 Combined treatment with dried leaves of Va in cadmium-induced rat further promote hypolipidemia, down regulated erythrocyte lipid profile, reduced cholesterol, triglyceride and increased phospholipid in the brain and liver respectively.
The hypolipidemic effects of dried Va leaves in this study is similar to the reports of Owen et al.,28 that shows dried Va meal has lipid–lowering effects in broiler chickens fed finishers’ mash. Previous reports that correlate with the results of this study are Ajuru et al.29 that revealed decreased lipid profile of normal rats and Audu et al.30 that reported hypolipidemic effect in rabbits. The lowering effect of dried Va leaves have been attributed to its phytochemical constituents like flavonoids, tannins and saponins.28,31,32 This could be that Va assists in the oxidation of cholesterol and triglyceride level,30,33 which led to the changes in the distribution of lipids in the different compartments.34 The observed decreased of plasma, erythrocyte, brain and hepatic cholesterol due to cadmium induction and treatment with dried Va leaves could be ascribed to their effects on cholesterol metabolism, triggering decrease in cholesterol supply for cell division and reparative processes. The decrease in cholesterol might also be due to inhibition of lecithin-cholesterol acyltransferase activity -a rate-limiting enzyme in cholesterol synthesis pathway35 and activation of the acyl-CoA: cholesterol acyltransferase which catalyzes intracellular esterification of cholesterol.36 Hence, an efflux of the lipids from the intracellular to the extracellular fluid occur from the compromised function of the plasma membrane.37 Cholesterol is synthesized from fatty acids that are attached to the glycerol side chain of triglyceride, thus, decrease or increase in the triglyceride level often decreases or increases the synthesis of cholesterol from the liver.
The study also shows decreased triglyceride concentrations in plasma and erythrocyte with the administration of both cadmium and Va; increased in liver and brain triglyceride concentration by cadmium but reduction with dried Va leaves treatment. The decrease or increase could be a consequence of lower uptake, higher efflux, increased degradation, decreased synthesis or a combination of these factors as evidenced by Alvarez et al.38 The lipid modulating property of the dried Va leaves on the animals as revealed by Ijeh and Ejike,12 Adaramoye et al.,32 might be due to the decrease or increase of the triglyceride concentration. The increased triglyceride concentration in the brain compartment with dried Va leaves treatment is consistent with the research of Spencer et al.,39 which may be as a result of increased activities of lipopropein lipase and triglyceride lipase that is associated with hypertriglyceridemia.40,41 The present study also revealed that cadmium and dried Va leaves treatment decreased plasma phospholipid, increased brain and liver phospholipid; while cadmium reduced erythrocyte phospholipid, the administration of dried Va leaves causes up/down regulation. In agreement with Miyahara et al.,42 El-Sharaky et al.,16 and Ugbaja et al.,37 the decreased phospholipid concentration could be through activation of hydrolyzing enzyme-phospholipase A2. The mechanism of action of dried Va leaves for lipid dyslipidemia properties dwell in the presence of flavonoid which was known to regulate fatty acid and cholesterol metabolism.43,44 Also, presence of saponins that precipitate cholesterol from micelles and interfere with enterohepatic circulation of bile acids, and thereby make cholesterol unavailable for intestinal absorption and by inhibiting pancreatic lipase activity, and reduce plasma triacylglycerol concentrations.44-46 The up-and-down lipid metabolism as observed in this research suggested that the membrane function is disrupted by cadmium-induction, and dried Va leaves as indicated in the cholesterol/phospholipid ratio.
These results shows that treatment with dried Va leaves has significant changes on the plasma, erythrocyte and organ lipid profile of the cadmium-induced rat.
There is no funding.
The authors gratefully acknowledge the technologies at the Tissue Culture Research Laboratory (Drug Discovery Unit), Department of Biochemistry, Lagos State University, Ojo, Lagos, Badagry Expressway, Lagos - Nigeria.
The authors declares that there is no conflict of interest.

©2019 Ogunrinola, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.