MOJ
eISSN: 2379-6294


Research Article Volume 4 Issue 2
1Maestría en Ciencia y Tecnología Química, Universidad Autónoma de Zacatecas, México
2Unidad Académica de Medicina Humana, Universidad Autónoma de Zacatecas, México
3Instituto de Biología Molecular en Medicina y Terapia Génica, Universidad de Guadalajara, México
4 Instituto de Investigación en Odontología, Universidad de Guadalajara, México
5Instituto Mexicano del Seguro Social, México
6 Laboratorio de Mutagénesis, Instituto Mexicano del Seguro Social, México
Correspondence: Ana Lourdes Zamora Perez, Instituto de Investigacion en Odontologia, Centro Universitario de Ciencias de la Salud, Universidad de Guadalajara, Jose Maria Echauri y Juan Diaz Covarrubias s/n, Col. Independencia Guadalajara, Jalisco, C.P. 44340, Mexico, Tel 0133 1058 5200
Received: March 09, 2018 | Published: April 3, 2018
Citation: Lazalde-Ramos BP, Zamora-Perez AL, Gutiérrez-Hernández R, et al. DNA protective effect of rosmarinus officinalis total extract in mouse peripheral blood. MOJ Toxicol. 2018;4(2):75–79. DOI: 10.15406/mojt.2018.04.00093
Rosmarinus officinalis is a common household aromatic plant widely used in cosmetic, food and folk medicine. The aim of this study was evaluated rosmarinus total extract (RTE) protective effect on DNA damage induced by cyclophosphamide (CP) and evidence the lack of genotoxicity and cytotoxicity of RTE in peripheral blood erythrocytes of Balb-C mice using the micronucleus assay. To evaluate the DNA protective effect, the dose of 100mg/kg of RTE was used and given orally. Following was the admiration of CP (50mg/kg). To evaluated the lack genotoxic or cytotoxic, three doses (30, 100 and 300mg/kg) of RTE were administered orally. A drop of blood was obtained from the tip of the tail of each mouse 24h for six days. We found no increase of micronucleated erythrocytes (MNE) or micronucleated polychromatic erythrocytes (MNPCE). Nor did polychromatic erythrocytes (PCE) decline in mice treated with one of the three different doses of RTE. RTE extracts were capable of diminished DNA damage caused by CP thus reducing MNE and MNPCE frequencies. The lack of genotoxic and cytotoxic effect of the RTE and reduces the genotoxicity caused by CP, suggest the potential therapeutic usefulness of this plant extract.
Keywords: genotoxicity, cytotoxicity, micronucleus, DNA protective effect, rosmarinus total extract
The DNA molecule is one of the main targets of the attack of free radicals in the cell generating different types of oxidative damage. There is a wide variety of plants that contain antioxidants that can counteract the oxidative damage to DNA within which Rosmarinus officinalis (rosemary) is found. Rosemary is a common evergreen aromatic perennial herb belonging to family Lamiaceae and grows wild in most Mediterranean countries Erkan et al.1 Rosemary extract, especially aerial parts, are used as a flavoring and anti-oxidant agent in food and beverage and in cosmetic industry Medicherla et al.,2,3 Rosemary is appreciated for its therapeutic in folk medicine uses for example as antidepressant, hepatoprotector, antidiabetic, antiangiogenic, anti-inflammatory and antitumor.4–8 Rosemary contains a complex mixture of phenolic antioxidants, including carnosol, carnosol acid and rosmarinic acid and are believed to be responsible for its pharmacological activity.9,10
The therapeutic properties attributed to plants are mainly responsible for the active principles it contains, and some poisoning and adverse reactions that can occur if used in inappropriate doses or for prolonged periods. Studies of medicinal plants in its preclinical phase include toxicology studies and within these are the genotoxicity tests.11–13 The in vivo micronucleus (MN) assay is a test used in screening genotoxicity and is recognized as part of product safety assessment.13 The MN are chromosomal fragments or whole chromosomes that spontaneously or because of clastogenic or aneuploidogenic agents, are excluded from the nucleus in mitosis.14 The MN formation leading to cell death, genomic instability, or cancer development.15 The mice peripheral blood MN assay is included in all testing batteries for genotoxicity and cytotoxicity safety evaluation and provides clear and accurate results Zúñiga-González et al.,16 Zamora-Perez et al.17 In this work, we evaluated the protective effect of R. officinales total extract (RTE) on DNA damage induced by cyclophosphamide (CP) in peripheral blood erythrocytes of Balb-C mice using the in vivo MN assay. As well was evaluated the genotoxic and cytotoxic potential of RTE to guarantee its safe use in humans.
Plant material and preparation of the extract
Dried leaves of R. officinales was obtained of Plantas Medicinales de América, S.A. de C.V. México, D.F., lot number 100210. The RTE was extracted as previously described.5 The dry leaves were pulverized into fine powder, macerated in methanol and an extraction in a reflux system for 2 h at 62 ºC was performed. The extraction was filtered under vacuum and was bleached with active carbon. A second extraction was performed to leaf residue with methanol and this extract was added to the first. The methanol extract was concentrated by distillation in a rotary evaporator. To this concentrated residue, distilled water was added which precipitated the RTE. Finally, the filtered precipitation was air-dried at room temperature to yield a fine yellow powder.18
Animals
All experiments were conducted with the approval of the local animal use regulatory body (Protocol register number UAZ-2008-35746) and carried out in full compliance with the guidelines for the care and use of experimental animals (Mexico; NOM-062-ZOO-2001) and international guidelines (Animal Welfare Assurance A5281-01). Thirty-five male Balb-C mice five to six weeks of age with average weight of 15.6±2.6 g were housed in a controlled environment with food and water ad libitum.
Groups and test agent treatment
Mice were distributed randomly into 7 groups of five animals each as shown in table 1. All doses were administered orally daily for two and/or five consecutive days and were adjusted to a final volume of 0.1mL per 10g weight. To evaluate the genotoxic/cytotoxic effect of RTE, five groups were formed using the following scheme: Group one received sterile water; Group two received 50mg/kg of CP (Sigma, St. Louis, MO; CAS No. 6055-19-2); Group three to five were given one of the three RTE doses (concentrations) (30, 100, or 300mg/kg bw., of RTE) (Table 1). The RTE doses were selected according as previously described Gutiérrez et al.5 To examine the effect of the RTE on DNA damage, a second experiment was performed as follows: Group six received 50mg/kg of CP and 100mg/kg of RTE separately (Table 1), and group seven was treated with 50 mg/kg of CP (Sigma, St. Louis, MO; CAS No. 6055-19-2) and 0.06mg/kg of folic acid (FA) Gómez-Meda et al.,19 (Sigma, St. Louis, MO; CAS No. 59-30-3). Separately, both CP and FA were dissolved in water and all doses were adjusted to a final volume of 0.1mL per 10g weight.
Treatment |
N |
Groups |
Doses |
Exposure Time |
Negative Control |
5 |
1 |
Water |
Every 24h/5 days |
CP |
5 |
2 |
50mg/kg bw |
Divided into two doses every 24h |
RTE 30 |
5 |
3 |
30mg/kg bw |
Every 24h/5 days |
RTE 100 |
5 |
4 |
100mg/kg bw |
Every 24h/5 days |
RTE 300 |
5 |
5 |
300mg/kg bw |
Every 24h/5 days |
RTE 100+CP |
5 |
6 |
As in (4) and (2) |
Every 24h/5 days + divided into two doses |
FA 0.06+CP |
5 |
7 |
0.06mg/kg bw and (2) |
Every 24h/5 days + divided into two doses |
Table 1 Experimental groups and treatment
All doses were administered orally daily and were adjusted to a final volume of 0.1 mL per 10 g weigh. Rosemay total extract (RTE) in group 6 and folic acid (FA) in group 7 were administered two hours after cyclophosphamide (CP).
Sample preparation and MN analysis
A drop of peripheral blood was taken from the tip of the tail of each mouse immediately before the treatment (0 hr. or basal value) and 24, 48, 72, 96, and 120 hr later. Two smears were made on cleaned microscope slides. The smears were air-dried, fixed in absolute ethanol for 10 min, and stained with acridine orange (Sigma; CAS No. 10127023).20 All slides were coded before microscopic analysis. The MN in each sample was scored manually using a microscope equipped with epifluorescence and a 100X objective. The number of MNE in 10,000 total erythrocytes (TE: normochromatic and polychromatic erythrocytes) and the number of MNPCE in 1,000 PCE, and the proportion of PCEs in 1,000 TE were evaluated.
Statistical analysis
The animal was used as the experimental unit for the statistical tests. Results are expressed as the mean± standard deviation of MNE, MNPCE, and PCE frequencies. The data were evaluated using the Statistical Program for Social Sciences (SPSS, v11.0) for Windows medical pack (SPSS, Chicago, IL). After establishing the normal distribution of the data, a parametric test was performed. Comparisons were made between each treatment group and their respective basal value (0hr.) by means of repeated measures one-way-ANOVA, followed by a Bonferroni test to correct the significance values of the multiple post hoc pairwise comparisons. A P-value of <0.05 was considered significant.
Evaluation of the genotoxic/cytotoxic effect of RTE
The frequencies and comparison of MNE, MNPCE and PCE values in peripheral blood samples from mice treated with different doses of RTE are shown in Table 2. Comparisons showed that negative control and experimental groups at the three doses of RTE tested did not show a statistically significant difference in MNE, MNPCE or PCE frequency, during the sampling period. However, the dose of 100mg/kg of RTE presented significant decrease of MNPCE frequency from 48hours to 120hours (Table 2). Moreover, the group who received the dose of CP (positive control) increased the frequency of MNE and MNPCE and decreased the proportion of PCE (Table 2).
Sampling time |
||||||||
Groups |
n |
|
0h |
24h |
48h |
72h |
96h |
120h |
Negative control |
5 |
PCE/1,000 TE |
39.00±9.46 |
42.20±10.02 |
39.40±11.90 |
37.02±9.17 |
34.80±10.11 |
35.20±9.81 |
P value |
NS |
NS |
NS |
NS |
NS |
|||
MNPCE/1,000 PCE |
6.80±1.30 |
7.80±2.58 |
5.40±3.87 |
6.00±5.56 |
5.80±3.42 |
4.20±2.38 |
||
P value |
NS |
NS |
NS |
NS |
NS |
|||
MNE /10,000 TE |
43.20±7.33 |
50.80±8.75 |
45.80±9.13 |
48.45±8.54 |
046.38±11.52 |
51.40±9.44 |
||
P value |
|
NS |
NS |
NS |
NS |
NS |
||
Positive control |
5 |
PCE/1,000 TE |
45.40±9.88 |
35.16±10.11 |
17.21±9.84 |
15.32±9.56 |
20.32±11.09 |
31.56±8.65 |
P value |
NS |
0.01 |
0.01 |
NS |
NS |
|||
MNPCE /1,000 PCE |
6.30±4.84 |
8.20±3.03 |
18.80±4.49 |
19.4±6.10 |
7.8±2.49 |
9.00±3.56 |
||
P value |
NS |
0.001 |
0.01 |
NS |
NS |
|||
MNE /10,000 TE |
42.60±9.63 |
56.18±8.12 |
63.80±7.42 |
75.21±5.74 |
58.80±8.44 |
52.60±6.75 |
||
P value |
|
NS |
0.01 |
0.01 |
NS |
NS |
||
RTE |
5 |
PCE/1,000 TE |
34.20±12.63 |
36.45±9.01 |
45.32±12.35 |
40.34±9.47 |
33.59±10.89 |
38.57±9.73 |
P value |
NS |
NS |
NS |
NS |
NS |
|||
MNPCE /1,000 PCE |
6.20±4.02 |
6.20±4.78 |
4.80±3.09 |
4.40±3.07 |
3.60±2.14 |
3.20±2.30 |
||
P value |
NS |
NS |
NS |
NS |
NS |
|||
MNE /10,000 TE |
41.00±9.4 |
37.80±8.82 |
46.40±11.31 |
46.12±9.50 |
51.40±10.87 |
44.33±9.57 |
||
P value |
|
NS |
NS |
NS |
NS |
NS |
||
RTE |
5 |
PCE/1,000 TE |
37.80±14.09 |
35.90±12.47 |
45.58±9.87 |
40.66±11.28 |
38.78±9.27 |
38.24±10.38 |
P value |
NS |
NS |
NS |
NS |
NS |
|||
MNPCE /1,000 PCE |
10.20±3.34 |
8.80±4.65 |
5.40±3.18 |
3.25±2.41 |
3.40±1.54 |
3.00±1.70 |
||
P value |
NS |
0.01 |
0.009 |
0.005 |
0.004 |
|||
MNE /10,000 TE |
47.20±9.25 |
44.40±7.42 |
47.60±9.68 |
49.40±8.67 |
40.60±8.53 |
40.80±7.46 |
||
P value |
NS |
NS |
NS |
NS |
NS |
|||
5. RTE |
5 |
PCE/1,000 TE |
33.0±18.16 |
58.40±11.54 |
53.40±16.00 |
43.20±9.80 |
36.00±10.70 |
39.80±12.10 |
P value |
NS |
NS |
NS |
NS |
NS |
|||
MNPCE /1,000 PCE |
6.83±2.54 |
7.20±3.11 |
4.80±2.86 |
4.21±3.12 |
4.34±2.91 |
4.20±2.78 |
||
P value |
NS |
NS |
NS |
NS |
NS |
|||
MNE /10,000 TE |
48.00±12.04 |
50.00±7.14 |
43.00±10.48 |
55.15±9.68 |
48.20±8.40 |
40.80±9.12 |
||
P value |
|
NS |
NS |
NS |
NS |
NS |
||
Table 2 Absence of cytotoxic and genotoxic effect of three different doses the rosemary total extract in mice
Data are expressed as mean±SD. Comparisons were made between different time points of the treatment groups and their respective basal values (0 hr).
Abbreviations: NS, no significant; RTE, rosemary total extract; CP, cyclophosphamide; TE, total erythrocytes; PCE, polychromatic erythrocyte; MNPCE, micronucleated polychromatic erythrocytes; MNE, micronucleated erythrocytes.
DNA protective effect of rosmarinus officinalis total extract
Mice were treated with CP in the presence and the absence of 100mg/kg of RTE or 0.06mg/kg of FA during five days (Figure 1). The group who received RTE 100mg/kg during five days simultaneously with 50 mg/kg dose of CP divided in two days showed a minor decrement of the proportion of PCE compared with the group who received CP, this difference was significant at 48 hours of sampling (P=0.01). It was observed a significant decrease in the number of MNPCE (P=0.001) at 48 and 96 hours in the group who received RTE and CP compared with the group that received CP alone. In addition, this group presents a smaller increase in MNE frequency that the CP group. This difference was significant at 72 and 120 hours (Figure 1). In addition, the group receiving the dose of CP and FA presented a minor decrease in the proportion of PCE and a minor increase in MNE and MNPCE frequency in comparison to the group that receives CP (Figure 1). In relation to the proportion of PCE and MNE or MNPCE frequency, no statistical difference was found between the groups of CP+FA and CP+RTE.
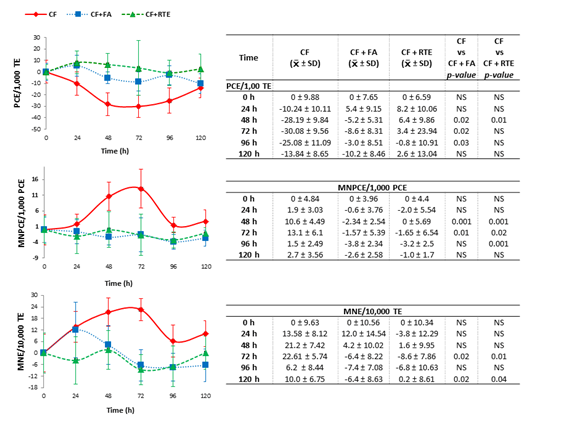
Figure 1 Proportion of micronucleated polychromatic erythrocytes (MNPCE), micronucleated erythrocytes (MNE) and polychromatic erythrocytes (PCE) in peripheral blood of mice treated with cyclophosphamide (CP) simultaneously with rosemary total extract (RTE) or folic acid (FA). TE, Total erythrocytes.
This study was designed to investigate the protective effect of methanolic extract of Rosmarinus officinalis on induced DNA damage by a mutagenic as is the case of CP, in vivo, also was evaluated the genotoxic and cytotoxic potential of RTE to ensure its safe use in humans. Genotoxicity and cytotoxicity of the different doses of RTE was measured using MN assay in mouse peripheral blood. Since peripheral blood is the first site of xenobiotics, it is biologically relevant to determinate DNA damage in this cellular type Larangeira et al.21 MN is a cytogenetic assay based on counting MN in dividing cell population. It also demonstrated chromosomal damage and it is a marker of genomic damage Hayashi et al.15 Our results show that oral administration of different doses of RTE did not induced genotoxic or cytotoxic effect in mice peripheral blood. As it was expected, the group who received the dose of CP (positive control) increased the frequency of MN in normochromic and polychromatic erythrocytes and decreased the proportion of PCE (Table 2), thus demonstrating the validity of the experimental design. Our findings are in accordance with the study of Gaiani et al.,22 who found that R. officinalis hydro-alcoholic extract at doses of 6.43, 100 and 200mg/kg body weight, did not induce statistically significant increases on MN frequency and MNPC frequency was increased but without significance. This differs from our results, since the three doses used in our study decreased MNPCE frequency, this could be explained, since it has been reported that RTE contains many substances like phenols with antioxidant and anti-lipoperoxidant activity Erkan et al.,1 Wang et al.23 The literature mentions that the combination of two acid-phenols, leads to an increase in antioxidant efficacy activity.24 In addition, phenolic compounds may also indirectly act as antioxidants in cells by modulating the activity of antioxidant, detoxifying and repairing enzymes as well as enzymes involved in the bioactivation of xenobiotics Ferguson et al.25
On the other hand, some studies describe that rosemary extracts supplementation present potential positive health effects. Singletary and Nelshoppen, observed that dietary supplementation with rosemary extract with its individual antioxidative constituents resulted in a significant decrease in carcinogen DNA adduct formation in mammary epithelial formation Singletary & Nelshoppen.26 In this study, the potential of RTE to reduce CP induced DNA damage was evaluated. As it shows in Figure 1, the dose of 100mg/kg of RTE protect against the mutagenic effects of CP. This decrease in MN demonstrated an antigenotoxic effect of RTE due to its antioxidant activity.1 Also our findings are according with those reported by different authors. Furtado et al. Furtado et al.,27 reported the capacity of rosmarinic acid (natural phenolic compound of rosemary) to reduce the frequency of doxorubicin to induced MNPCE. Pereira et al.28 also found no DNA damage induced by rosmarinic acid in peripheral blood Wistar rat using the comet assay. Vattem et al.,29 demonstrated the antimutagenic activity of rosmarinic acid in the Ames test using Salmonella typhimurium, in which rosmarinic acid inhibited the mutagenic potential of sodium azide and N Methyl-N-nitro-N-nitrosoguanidine and they concluded that the antimutagenic activity of rosmarinic acid might be due to modulation of the redox environment in the bacterial cell. Del Baño et al.,30 reported that carnosic acid and carnosol are compounds with antimutagenic activity both before and after gamma-irradiation treatments. The inhibitory effect of rosmarinic acid was associated with the up regulation of superoxide dismutase and glutathione and its scavenging effect on free radicals Kin et al.,31 Fahim FA et al.,32 observed hepatoprotective and antimutagenic activities of the rosemary ethanolic extract and essential oil, respectively, and according with the authors, these effects are attributed to the presence of a relatively high percentage of phenolic compounds with high antioxidant activity.
In conclusion, our results reveal that by means of MN assay in mouse peripheral blood erythrocytes, methanol extract of leaves of R. officinalis did not induce genotoxic or cytotoxic effect. By contrast, it shows DNA protective effect, to decrease DNA damage induced by cyclophosphamide and increasing the evidence about the antioxidant properties attributed to this plant. The absence of genotoxic and cytotoxic damage of RTE, as well as the antigenotoxic activity indicate the potential dietary and therapeutic safe usefulness of this extract.
None.
The authors declare no conflict of interest.

©2018 Lazalde-Ramos, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.