MOJ
eISSN: 2379-6294


Pyocyanin, the growth pigment produced by Pseudomonas aeruginosa, thought at one time to be a metabolic byproduct, was found to be important for the wellbeing of the organism and one of the important chemicals that allow it to survive in environments with competitive organisms. Different methods were used to extract and purify pyocyanin, some requiring major instruments. This study was performed to introduce an amended method for the optimal extraction and purification of pyocyanin. The results showed that the maximum yield was obtained from Cetrimide Agar plates seeded with the organism and held at room temperature for 96 hrs. The purified pyocyanin’s rate of degradation was dependent on the concentration of the sodium hydroxide added, its optimal storage solvents were deionized water and/or 80% methanol, but not dry chloroform in which the pigment quickly degraded. The purified pyocyanin was found to be thermolabile as it was stable for many days at lower temperatures but was immediately inactivated by higher temperatures. The suggested method is easy to implement, requires only regular laboratory facilities and gives a good yield of the pure pigment. Recommendations are also provided for proper storage to maintain its activity for a considerable period of time.
Keywords: Pyocyanin, extraction and purification method, bacterial pigment, Pseudomonas aeruginosa.
Pseudomonas aeruginosa is an aerobic Gram negative, rod shaped bacterium with special features making it one of the most commercially and biotechnologically valuable microorganisms.1,2 One of the hallmarks of P. aeruginosa is its ability to produce the blue-green pigment pyocyanin. Pyocyanin belongs to the family of phenazine which are, by definition, heterocyclic compounds that are produced naturally, but with side chains substituted at different points around their rings by different bacterial species.3 The biosynthesis of pyocyanin starts from a phenazine produced naturally: the deep red 5-methyl-7-amino-1-carboxyphenazium betaine that is converted to phenazine-1-carboxilic acid, which is lemon yellow in color. This is the last precursor of the bright blue 1-hydroxy-5-methyl phenazine or pyocyanin.4 Genetic analysis and gene expression assays showed that in P. aeruginosa, 7 genes are responsible for the synthesis of pyocyanin. These genes include phz C, D, E, F, G, M and S, but it was shown that phzM and phzS were the primary genes responsible for converting phenazine-1-carboxylic acid to pyocyanin through the production of S-adenosyl methionine-dependent N-methyltransferase and flavin-dependent hydroxylase respectively.4 The biosynthetic loci of the phenazine were found in all P. aeruginosa species (Figure 1).4–6 Pyocyanin is characterized by its blue color, but, its color and characteristic absorption spectrum are pH-sensitive. In fact, the strong blue color is only detected in neutral or basic pH, whereas under acidic conditions, pyocyanin becomes red in color.7 Although pyocyanin is a blue pigment when isolated in neutral pH, its presence in agar plates is detectable by the appearance of a blue-greenish color. This is due to the interference between the blue pigment and the color of the medium.
Originally, it was though that pyocyanin was just a secreted growth pigment, without any biological function and was considered as a metabolic byproduct of P. aeruginosa.8 However, it was discovered later that pyocyanin was a redox-active compound capable of donating and accepting electrons. For as pyocyanin could cross biological membranes easily, it served as a mobile electron carrier for P.aeruginosa. The colorless, reduced pyocyanin donated one electron to molecular oxygen, which is the main electron acceptor under aerobic conditions, thereby creating a superoxide anion causing its color to change to the blue oxidized form.9 In addition, pyocyanin plays a major role in the survival of P. aeruginosa under oxygen-poor conditions. In fact, pyocyanin can accept electrons generated from NADH in carbon source oxidation and transport the electrons away from the bacterium to acceptors found at remote places.10 Moreover, according to Jayaseelan and his colleagues (2013), pyocyanin is involved in a variety of biological activities including quorum sensing, maintaining fitness of bacterial cells and biofilm formation.11 Pyocyanin was also shown to exhibit antibacterial and antifungal properties.12,13 In addition, in one of our ongoing projects, pyocyanin was shown to inhibit biofilm formation of many clinically significant bacteria.14 This study aimed at finding a simple and practical method for the extraction and purification of pyocyanin from P. aeruginosa.
The P. aeruginosa isolates used in the study were clinical isolates courteously provided by the clinical microbiology laboratory of the Lebanese American University Medical Center- Rizk Hospital (LAUMC- RH). The identity of the isolates was confirmed by standard methods.15
Amended method for the aqueous extraction and purification of pyocyanin
Since this is an amended procedure for the aqueous extraction and purification of pyocyanin, it is presented in steps for the sake of clarity:
Table 1 and Figure 3 show the amount of pyocyanin produced by P. aeruginosa on agar plates held for variable periods of time. It was obvious that the amount of pyocyanin extracted was dependent on the time the plates were left at room temperature. The reported time is the time spent by P. aeruginosa on the agar plates, from step 4 to step 6. The amount of pyocyanin produced was quantified based on the volume of pure pyocyanin extracted. Results showed that pyocyanin extraction yielded the highest volume from the plates held for 96hrs., followed by those held at 72hrs., 168hrs., 240 hrs. and finally those held for 48 hrs. It was also found, as is clear from Table 2 and Figure 4, that the rate of pyocyanin degradation was dependent on the concentration of NaOH [NaOH] added. In fact, results showed that the lower the concentration of NaOH added, at step 19, the lower was the degradation rate of pyocyanin and therefore the longer pyocyanin was in its active form and could be used. When the concentration of NaOH was 0.005 mM, pyocyanin lasted for 30 days in its active form, whereas as the concentration of NaOH increased, the longevity of pyocyanin decreased to as little as 1 day when the concentration reached 0.2 M [NaOH].
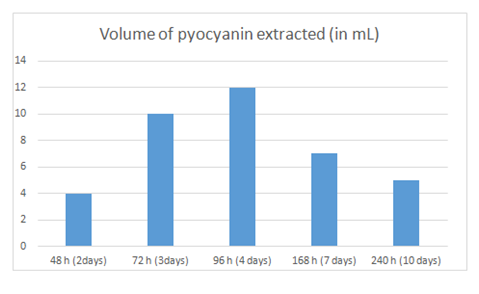
Figure 3 Graphic representation of the data in Table 1. X axis: time that elapsed between seeding P. aeruginosa on Cetrimide Agar plates (step 4) and its removal (step 6).; Y axis: amount of pyocyanin produced represented by the volume of pyocyanin extracted in pure form (in mL).
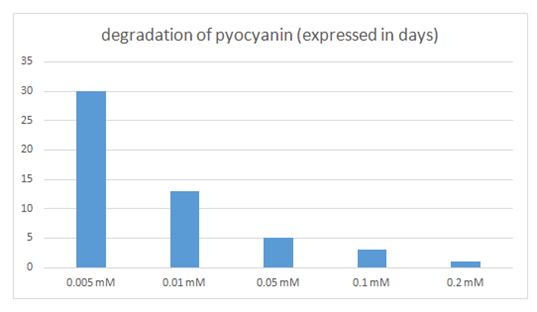
Figure 4 Graphic representation of the data in Table 2. X axis: concentration of NaOH ([NaOH]) added; Y axis: time in days taken to degrade pyocyanin.
|
48 hrs.(2 days) |
72 hrs.(3 days) |
96hrs.(4days) |
168 hrs.(7 days) |
240hrs.(10days) |
Volume of pyocyanin extracted |
4mL |
10mL |
12mL |
7mL |
5mL |
Table 1 Amount of pyocyanin produced, expressed by the volume of pure pyocyanin extracted, as per the time that elapsed between seeding P. aeruginosa on Cetrimide Agar plates (step 4) and its removal (step 6)
Concentration of NaOH added (in mM) |
0.005 mM |
0.01 mM |
0.05 mM |
0.1 mM |
0.2 mM |
degradation of pyocyanin (expressed in days) |
30days |
13days |
5days |
3days |
1day |
Table 2 Time in days taken by pyocyanin to be degraded, when the concentration of NaOH (mM]) was varied
Furthermore, the nature of the solvent in which pyocyanin was stored, affected its stability over time given that all solvents contained the same starting concentration. In fact, as reported in Table 3 and Figure 5, the stability of pyocyanin in 80% methanol was comparable to its stability in water given the same concentration of solutes, whereas dry chloroform stimulated the degradation of pyocyanin when acting as a solvent for a long period of time. Pyocyanin was found to last for only 2 days in dry chloroform before it was degraded as compared to 12 days in water ([HCl] = [NaOH] = 0.1 M). On the other hand, pyocyanin was shown to be thermolabile. Table 4 and Figure 6 show that the stability of pyocyanin was dependent on the surrounding temperature. It was evident that pyocyanin, same concentration considered, was degraded progressively with increasing temperatures. In fact, pyocyanin was stable for 12 days at a temperature of 25oC, whereas it was stable for only 1 day at a temperature 70oC (in a water bath).
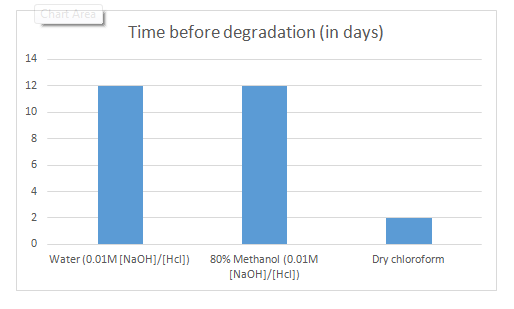
Figure 5 Graphic representation of the data in Table 3. X axis: nature of the solvent; Y axis: time in days taken to degrade pyocyanin.
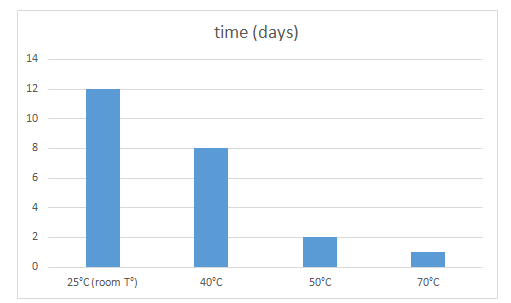
Figure 6 Graphic representation of the data in Table 4. X axis: surrounding temperature (oC); Y axis: time taken, in days, to degrade pyocyanin.
Solvent |
Water(0.01M [NaOH]/[Hcl]) |
80%Methanol(0.01M [NaOH]/[Hcl]) |
Dry chloroform |
Time before degradation (in days) |
12days |
12days |
2days |
Table 3 Period of stability of pyocyanin, in days, in the different solvents tested
temperature (°C) |
25°C (room T°) |
40°C |
50°C |
70°C |
time(days) |
12days |
8days |
2days |
1day |
Table 4 Period of stability of pyocyanin, in days, in different surrounding temperatures (0C)
In this study, pyocyanin was extracted by introducing amendments to the method previously described by Wilson et al. (1987).16 These amendments included growing P. aeruginosa on Cetrimide Agar instead of King’s A Agar, adjustment of the incubation and holding time to maximize pigment production, and adding a dry chloroform step to the acid/base extraction, to help optimize the final yield of pyocyanin. The procedure used in this study included two major steps: The first was based on the redox properties of pyocyanin and on the fact that pyocyanin changed its color when it passed from basic to acidic pH. These properties gave pyocyanin the special characteristic of having different solubilities based on its state: when in the red state, pyocyanin was soluble in aqueous solvents more than in organic solvents, whereas in its blue state, it was soluble in organic solvents more than in aqueous solvents. This duality in solubility made the purification of pyocyanin through changing the pH and water/chloroform extraction possible and highly effective. The second major step was the petroleum ether step (step 15 in the detailed method above), which was effective in purifying pyocyanin and increasing its yield. In fact, the crystals formed by the addition of petroleum ether were formed of pure pyocyanin (observed in step 18 above, after the last acid/base extraction of the pigment from chloroform). The recovered chloroform then was transparent, as if not used, as compared to its color after the first acid/base extraction where it was lemon-yellow in color. Since only pyocyanin had the ability to change solubility with changing pH, it was concluded that the formed crystals were made up of pure pyocyanin. In addition, the formation of pyocyanin crystals was necessary to measure the mass of the recovered pigment in order to obtain the desired concentration by adjusting the volume of the solvent added.
To get the pyocyanin to crystalize, other strategies were considered and tried, but they turned out to be ineffective. First, the petroleum ether step was replaced simply by heating through putting the solution in a water bath and waiting for the chloroform to evaporate. This was a miscalculated approach, because once the chloroform evaporated and the crystals were recovered, it was not possible to separate the pigment from the impurities that crystalized with it. However, this attempt was also not successful because pyocyanin was getting degraded in the process. Since it was known that the boiling point of dry chloroform under normal conditions is 61.2°C, and with the additions of solutes (pyocyanin and some impurities) the boiling point increased, this delayed the evaporation of chloroform for days even when put at 70°C., but, and with reference to Table 4, it was shown that pyocyanin at 70°C was active only for one day. Furthermore, and referring to Table 3, pyocyanin in chloroform, as a solvent, remained active for only 2 days. So, these procedures were not successful in generating the desired output.
Another alternative was to use a rotary evaporator, as that would have been a way to evaporate chloroform at low temperatures by lowering the pressure. This seemed to be a perfect solution to the problems faced earlier, as it would evaporate chloroform using low temperatures and in a short period of time. As a matter of fact, after step 14, the chloroform containing pyocyanin was taken to the rotary evaporator and the chloroform did evaporate leaving the blue crystals intact. However, the crystals recovered were not water soluble although pyocyanin is a water-soluble pigment. These crystals remained water insoluble even after vigorous shaking at a temperature of 37°C overnight, however, it was noticed that these crystals were highly soluble in chloroform. After various unsuccessful trials, it was found that the crystals were not only formed of pyocyanin. In fact, chloroform was crystalizing with the pigment and trapping it, preventing its contact with water. A simple experiment was done where rotary evaporation was performed on dry chloroform: after the evaporation of chloroform, colorless crystals were recovered, proving the that that method was also not the one desired. Also, and aiming to of maximize the yield of pyocyanin, after step 8, the bottle containing the mixture was put in an orbital shaker at 40°C, hoping that by shaking for a long period of time, all the pigment will pass to the chloroform phase. However, with chloroform as a solvent (Table 3) and at 40°C (Table 4), pyocyanin was degraded within hours. The concentrations of the HCl and NaOH used in different steps were purposefully chosen. At step 11, in order not to keep pyocyanin for a long time in this phase, a high concentration of HCl (0.2M) was needed to turn all the pyocyanin into the red phase without adding too much volume (Table 2). Whereas at steps 18-19, the purpose of using the solvent was to store pyocyanin for longer periods of time while simultaneously achieving the desired concentration, a reason why the 0.01M concentration was used as it proved to be the most suitable and met the desired requirements of stability and concentration.
The results in Table 1, showed that extracting the pigment after 4 days was most effective than any other period of time as holding for a period other than that produced small amounts of pyocyanin making the extraction less productive. And even though extracting the pigment after 96 hours (4 days) gave more time to P. aeruginosa to produce more pyocyanin, yet it was found that the yield was lower since the water was evaporating from the agar plates causing the pigment to deteriorate. Furthermore, after a long holding time, P. aeruginosa would start producing other pigments like pyorubin (red-brown), pyomelanin (light brown) and pyoverdin (yellow, green and fluorescent) (Reyes et al. 1981; Meyer 2000).17,18 These pigments could have affected the production rate of pyocyanin and interfered with its extraction. In our numerous trials, it was found that incubating the plates at 35°C for 2 days then leaving the plates at room temperature for 2 more days gave a better pyocyanin yield than if the plates were incubated for 4 days at 35°C. A reason why when the pyocyanin yield over time was tested (Table 1), the incubation period for all was for 48 hrs at 35°C after which holding time at room temperature was varied. On the other hand, it was noticed that pyocyanin production by P. aeruginosa on Cetrimide Agar (CA) plates was higher than on Mueller Hinton Agar (MHA) plates. In fact, the volume of pyocyanin recovered from CA plates was higher than the volume that was recovered from MHA plates under the same conditions. The fact that pyocyanin production was dependent on the nature and composition of the agar was investigated by Sheeba (2007) and it was found that among six solid media used (Tryptone Glucose Yeast Agar, Seller’s Differential Medium, Nutrient Agar, Fluorescein Denitrification Agar, Cetrimide Agar and Pseudomonas agar), Cetrimide Agar showed maximal production of pyocyanin.19
Furthermore, the procedure used in this study was optimized and refined by repeating for more than 20 times on the same P. aeruginosa isolate. The extracted pigment was used in another ongoing project which aimed to prove the antibacterial and antibiofilm activity of pyocyanin against some Lebanese strains of clinically significant bacteria. Particularly, the information about the 80% methanol solvent was obtained from the procedure of testing the ability of pyocyanin to inhibit bacterial biofilm formation.14 Furthermore, this procedure was used on 3 different P. aeruginosa clinical isolates and the pyocyanin was optimally extracted from all strains, making the procedure easy to use for isolation of pyocyanin from any P. aeruginosa isolate.
This study showed that pyocyanin can be extracted using a simple but effective method. The pyocyanin pigment was extracted in pure form by following easy steps that were based on the chemical and physical properties of the compound itself. In this suggested procedure, pyocyanin was extracted without using big instruments, making the extraction of pyocyanin accessible and affordable to laboratories of all sizes.
None.
The authors declare that there is no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.