MOJ
eISSN: 2577-8374


Research Article Volume 1 Issue 2
1Inorganic & Physical Chemistry Division, CSIR-Indian Institute of Chemical Technology, India
2Academy of Scientific and Innovative Research (AcSIR), India
3Environment and Sustainability Institute (ESI), University of Exeter, UK
4Department of Mechanical, Brunel University, UK
Correspondence: Senthilarasu Sundaram, Lecturer in Renewable Energy, Environment and Sustainability Institute, University of Exeter, Penryn, Room No: 01.27, Cornwall TR10 9EZ, UK, Tel 44 (0) 1326 259486
Co-correspondence: Lingamallu Giribabu, Inorganic & Physical Chemistry Division, CSIR-Indian Institute of Chemical Technology, Uppal
Road, Tarnaka, Hyderabad-500007, Telangana, India,
Received: September 27, 2017 | Published: October 6, 2017
Citation: Jella T, Selvaraj P, Upadhyaya HM, Mallick TK, Senthilarasu S et al. (2017) Synthesis and Photo Electrochemical Characterization of an Extended ?-Conjugated Heteroleptic Ruthenium (II) Complex. Open Acc J Photoen 1(2): 00007. DOI: 10.15406/mojsp.2017.01.00007
A new extended π-conjugate heteroleptic ruthenium(II) complex (m-HRD-1) that contains a 4,4'-bis-2-(5(3,5-di-tert-butylphenyl)thiophene-2-yl)vinyl)2,2'-bipyridine as ancillary ligand, 4,4’-dicaboxy-2,2'-bipyridine as anchoring group, and two thiocyanate ligands in its molecular structure have been designed, synthesized and characterized by CHN, Mass, 1H-NMR, UV-Vis, and fluorescence spectroscopies as well as cyclic voltammetry. Electrochemical and theoretical studies showed that the LUMO of the sensitizer is above TiO2 conduction band and the HOMO is below the redox potential of the electrolyte. This new sensitizer was tested in dye-sensitized solar cells using liquid redox couple (I-/I3-) and its performance was compared to the standard sensitizer N719.
Keywords: dye sensitized solar cells, extended p-conjugation, bipyridine, heteroleptic, redox electrolyte
DSSC, dye-sensitized solar cell; IR, infrared; CV, cyclic voltammogram; PV, photovoltaic; Jsc, short circuit current density; Voc, open circuit voltage; FF, fill factor; DTG, differential thermogravimetr
Dye-Sensitized Solar Cells (DSSCs) are one of the rising hopes for the future renewable energy sources among the excitonic photovoltaics because of their encouraging power conversion efficiencies of >10% for metal free dyes, >11% for metal complex and 13% for porphyrin based sensitizers.1–6 As sensitizers take part in the absorption of solar radiation and injection of electrons into the conduction band of semiconductors, extensive research has been focused on the design of efficient and durable sensitizers. Several dyes have been investigated such as metal complexes, metal-free organic dyes, tetra pyrrolic porphyrin and phthalocyanine based sensitizers.7–16 Among them, the most successful charge transfer sensitizers are ruthenium based dye molecules, because of their strong and broad metal to ligand charge transfer absorption bands, the stability of their oxidized form and longevity of photo excited states. Grätzel and co-workers have reported >11% efficiency with cis-di(thiocyanato)-bis [2,2'-bipyridyl 4,4'-dicarboxylic acid] ruthenium(II)(N3) and trithiocyanato-4,4'4"-tricarboxy-2,2':6',2"-terpyridine ruthenium(II) (the black dye) as sensitizers.17 Many efforts have been made to design the ligands of ruthenium complexes to improve the spectral response to near IR region and device performance.
By substituting the two long alkyl chains on the bipyridyl ligand (Z907), Zakeeruddin and co workers achieved impressive stability under both thermal and light conditions.18 However, the molar extinction coefficient of sensitizer is somewhat lower than that of the standard N-719 dye. Thus, extended π-conjugation has been introduced into the ligands to improve the molar extinction coefficient. For instance, Z910, which contains 3-methoxystyryl on bipyridine ligand, exhibited 10.2% efficiency and remarkable stability.19 Based on these conclusions several dyes have been developed using different substituents on styrene moiety of bipyridyl ligand- K19, K77, HRD and electron donors on bipyridine ligands such as triphenylamine, carbazole and coumarin etc.20–24 By using electron donating substrates charge recombination decreased drastically, as this forms long charge separated state lifetimes. Wu and co-workers incorporated alkyl thiophene substituted bipyridine as an ancillary ligand on the ruthenium metal complex (CYC-B1, C101), as a consequence, the MLCT band has shifted to the red region and the energy levels of metal center can be raised for better charge injection and recombination.25,26
Our group is also engaged in the design and development of efficient heteroleptic Ru (II) polypyridyl complexes.22–29 For instance, we have introduced 3, 5-di-tert-butyl phenyl group on extended π-conjugation of bipyridine ligand (HRD-1). HRD-1 has shown a conversion efficiency of 5.77% and impressive performance in thermal and light soaking studie.22 The objective of this work is to re-design and synthesis HRD-1 complex by introducing thiophene moiety between the 3,5-di-tert-butyl phenyl group, also extending π-conjugation of bipyridine ligand to get heteroleptic Ru(II) (m-HRD-1) complex in order to further improve the absorption properties and also to tune the energy levels. Moreover, characterization of the complex by various spectroscopic techniques and its DSSC performance using liquid I-/I3- redox couple. The structure of m-HRD-1 and its ligand are shown in Figure 1.
All chemicals and solvents were purchased either from Sigma Aldrich or BDH (India) and purified prior to use. The compound 4, 4’-diethyl ester phosphonate-2,2’-bipyridine (2) was synthesized as per literature methods.27
Synthesis
Synthesis of 5-(3,5-di-tert-butylphenyl)thiophene-2-carbaldehyde(1): 3,5-di-tert-butyl bromobenzene (942 mg, 3.5 mmol) was dissolved in 40 ml of dry toluene, to which CsCO3 (393.6 mg, 1.2 mmol), Pd(PPh3)4 (0.25 equivalents) and 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)thiophene-2-carbaldehyde (1.86 g, 7.8 mmol) were added. The reaction mixture was then refluxed under nitrogen atmosphere for 12h. After cooling to RT, the crude mixture was purified using silica gel column EtOAc/Hex (1:4 v/v) to provide the desired product (90% yield). Elemental analysis of Anal. Calcd. For C19H24OS % (300.460): C, 75.95; H, 8.05; N, 5.32. Found: C, 75.92; H, 8.03; N, 5.30. ESI-MS (m/z): C19H24OS [300.460]: M 301 (100%). 1HNMR (CDCl3, δppm): 9.85 (s, 1H), 7.70 (m, 1H), 7.45 (m, 3H), 7.35 (m, 1H), 1.35 (s, 18H).
Synthesis of 4,4'-bis-2-(5(3,5-di-tert-butylphenyl)thiophene-2-yl)vinyl)2,2'-bipyridine (3): This ligand was synthesized by using Wittig-Horner’s reaction.30 NaH (360 mg, 15 mmol) was added to a solution of 2,2’-bipyridine-4,4’-diphosphonate (1.5 g, 3.5 mmol) and 5-(3,5-di-tert-butylphenyl)thiophene-2-carbaldehyde (1) (0.51 g, 7.8 mmol) in 150 ml of dry tetrahydrofuran (THF). The resulting mixture was refluxed overnight under nitrogen atmosphere. The reaction mixture was allowed to cool to room temperature and filtered the compound. The filtrate was concentrated and the solid was washed with methanol and dried to obtain the desired product in pure form of 75% yield. Elemental analysis of Anal. Calcd. For C50H56N2S2 % (749.132): C, 80.17; H, 7.54; N, 3.74. Found: C, 80.20; H, 7.52; N, 3.70. ESI-MS (m/z): C50H56N2S2 [740.132]: M 750 (100%). 1H NMR (CDCl3, δppm): 8.65 (d, 2H), 8.50 (s, 2H), 7.55 (d, 2H), 7.42 (s, 4H), 7.35 (s, 2H), 7.32 (d, 2H), 7.20 (d, 2H), 7.15 (d, 2H), 6.95 (d, 2H), 1.42 (s, 36H).
Synthesis of Ru(L)(p-cymene)(Cl)2: A mixture of 3 (0.38 g, 1.25 mmol) and [Ru(Cl)2-(p-cymene)]2 in ethanol: chloroform (8:2 v/v) was refluxed for 4 hours under nitrogen atmosphere. Evaporation of the solvent under reduced pressure produced the pure complexes as an orange solid.
Synthesis of m-HRD-1: The above p-cymene complex (1.24 mmol) and 4, 4’-dicarboxy-2, 2’-bipyridine, (L) (0.303 g, 1.24 mmol) in anhydrous DMF (75 ml) were heated to 140 °C for 4 hours under nitrogen atmosphere and in the dark. NH4SCN (1, 5 g, 19.7 mmol) was then added to the mixture and heating was continued for 4h. After cooling to room temperature, DMF was evaporated and water was added. The resulting purple solid was filtered and washed with water. The crude complex in basic methanol [with tetrabutyl ammonium hydroxide (TBAOH)] was further purified on a Sephadex LH-20 column with methanol as eluent. The main band was collected, concentrated, and precipitated with dilute acidic methanol to obtain pure desired complex. Elemental analysis of Anal. Calcd. For C80H99N7O4RuS4 1(TBA) % (1452.02): C, 66.17; H, 6.87; N, 6.75. Found: C, 66.20; H, 6.20; N, 6.72. ESI-MS (m/z): C80H99N7O4RuS4 1(TBA) [1452.02]: M+1 1453 (5%).
Characterization methods
The optical absorption spectra were recorded on a Shimadzu (Model UV-3600) spectrophotometer. Steady state fluorescence spectra were recorded using a Spex model Fluorlog-3 spectrofluorometer for solutions having optical density at the wavelength of excitation (lex) » 0.11. 1H NMR spectra were obtained at 300 MHz using a Brucker 300 Avance NMR spectrometer running X-WIN NMR software. The chemical shifts are related to tetramethylsilane (TMS). The Fourier transform IR (FTIR) spectra of all the samples were measured using a Thermo Nicolet Nexus 670 spectrometer. Cyclic- and differential pulse voltammetric measurements were performed on a PC-controlled CH instruments model CHI620C electrochemical analyzer. Cyclic voltammetric experiments were performed in 1 mM dye solution in acetonitrile at a scan rate of 100 mV/s using 0.1 M tetrabutyl ammoniumperchlorate (TBAP) as a supporting electrolyte. A glassy carbon electrode, a standard calomel electrode (SCE), and a plantinum wire were used as the working electrode, reference electrode and counter electrode respectively. After a cyclic voltammogram (CV) had been recorded, ferrocene was added, and a second voltammogram was measured. Thermogravimetric measurements were carried out on a Mettler Toledo TGA/SDTA 851e instrument at a heating rate of 10°C min-1 with 10 mg of sample under nitrogen atmosphere.
Computational methodlogy
Ground state geometries optimized in the gas phase with the PBEO [31] functional with LanL2DZ32–34 with effective-core potential (ECP) were used for Ru, and for rest of the elements 6-311+G (d,p) was used.35,36 The conductor-like polarisable continuum model (C-PCM)37 was used to optimize the geometries in acetonitrile. TD-DFT (time-dependent density functional theory) calculations were performed on the PBE0 optimized geometries using CAM-B3LYP38 with the above mentioned mixed basis set to calculate the vertical excitation energies, since it has been previously reported by many researchers that the CAM-B3LYP functional gives reliable results for MLCT.39 All the calculations were done in Gaussian 09.40
Dye cell preparation
The detailed TiO2 photoelectrode (area: ca. 0.28 cm2) preparation was described in our earlier studies.41,42 Briefly, nanocrystalline TiO2 films of 8-8.5 m thickness were deposited onto transparent conducting glass (fluorine-doped stannic oxide layer, sheet resistance of 8-10 /cm2). Then a scattering layer of ~4 µm thickness of 400 nm anatase TiO2 particles was deposited by screen-printing method. These films were gradually sintered at 500°C for 30 min. The heated electrodes were impregnated with a 0.04 M titanium tetrachloride solution in water saturated desiccator for 30 min at 70 °C then washed with distilled water and ethanol. The electrodes were annealed again at 500 °C for 30 min and then allowed to cool to 50 °C before dipping them into the dye solution. The TiO2 electrodes were soaked overnight in an ethanolic solution of 1×10-6M N719 dye (cis-diisothiocyanato-bis(2,2ʼ-bipyridyl-4,4ʼdicarboxylato)ruthenium(II)bis(tetrabutylammonium)) (Solaronix SA), sandwiched with a platinised conducting counter electrode prepared on FTO substrate using a Surlyn frame (Solaronix SA), filled with the electrolyte through a hole in the counter electrode and sealed. The iodide/tri-iodide electrolyte comprising 0.4 M LiI, 0.4 M tetrabutylammonium iodide (TBAI), and 0.04 M I2 dissolved in 0.3 M N-methylbenzimidazole (NMB) in acetonitrile (ACN) and 3-methoxypropionitrile (MPN) solvent mixture at volume ratio of 1:1 was used. The photovoltaic performances of the assembled devices were meaured under 1000 W/m2 of light from a Wacom AAA continuous solar simulator (model: WXS-210S-20, AM1.5G).
The details of the synthetic strategy adopted for the synthesis of m-HRD-1 are shown in Figure 1. The compound 5-(3,5-di-tert-butylphenyl)thiophene-2-carbaldehyde was synthesized by adopting Suzuki coupling between 3,5-di-tert-butyl bromobenzene and 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)thiophene-2-carbaldehyde.43 Bpy-phosphonate was synthesized as per the literature methods.27 We have adopted Wittig-Horner's reaction for the introduction of C=C double bond was introduced at 4, 4’ positions of the bipyridine ligand using Bpy-phosphonate and 1.30 The ligand Bpy-thio-butyl (L) was characterized by various spectroscopic techniques that include elemental analysis, Mass, IR and 1H NMR spectroscopies (See Supporting Information). The ligand L and [RuL (p-cymene)Cl2]2 complex by refluxing in ethanol: chloroform mixture to get the chloro derivative of m-HRD-1 complex. Finally, the m-HRD-1 complex was synthesized by refluxing chloro derivative with Bpy-acid and aq. ammonium thiocyanate in DMF and following by spehadex column purification. Preliminarily, m-HRD-1 was characterized by elemental analysis and ESI-MS spectroscopies. The presences of a molecular ion peak at 1453 (m/z) in ESI-MS spectrum confirms one TBA molecule in its molecular structure (See Supporting Information).
Optical properties
Figure 2 reveals the absorption spectra of m-HRD-1 in ethanol and the corresponding data are presented in Table 1. The absorption bands between 450 to 550 nm regions can be ascribed to the metal to ligand charge transfer transitions in singlet manifold (1MLCT). The absorption maximum of m-HRD-1 is centered at 538 nm with a molar extinction coefficient of 15,338 M-1 cm-1. The MLCT band of m-HRD-1 is bathochromically shifted when compared to HRD-1, probably due to the extended π-conjugation. Intraligand -* transitions bipyridine ligand are located at 300 nm. We have also measured absorption spectra of m-HRD-1 on an opaque TiO2 film (6 m thick). The absorption spectra in solution and on TiO2 were similar except for a slight red shift in absorption maxima due to the interaction of anchoring groups to the nanocrystalline TiO2 surface. The emission spectrum of m-HRD-1 was measured in ethanol solvent at room temperature and presented in Figure 2. The complex m-HRD-1 has shown emission maxima at 755 nm, when excited at MLCT band of m-HRD- complex. We have observed quenched emission spectra, when m-HRD-1 complex adsorbed on a 6 m thick nanocrystalline TiO2 layer, as a consequence of electron injection from the excited state of Ru (II) complex to the conduction band of TiO2. From the absorption and emission spectra, the singlet state (E0-0) energy of m-HRD-1 and HRD-1 are 1.94 & 1.90 eV, respectively. Quenched emission spectrum of m-HRD-1 was observed when adsorbed.
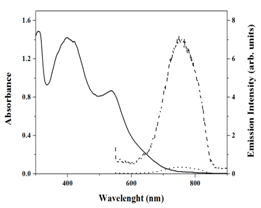
Figure 2 Electronic absorption (_______) and emission (-------) spectra of m-HRD-1 in ethanol solvent. Emission (.........) spectra m-HRD-1 adsorbed onto a 2 mm thick TiO2 film.
|
Sensitizer |
E1/2 V vs. SCEc Ox Red |
E0-0, eVd |
Eox* |
||
|
m-HRD-1 |
538 (15,338) |
755 |
0.67 -0.95 |
1.94 |
-1.27 |
|
HRD-1 |
543 (19,300) |
720 |
0.75 -0.94 |
1.9 |
-1.15 |
Table 1 UV-Visible, emission and electrochemical data
aSolvent: ethanol, Error limits: lmax, ±1 nm, e±10%.
bSolvent: ethanol, lmax, ±1 nm.
cSolvent: DMF, Error limits: E1/2±0.03 V, 0.1 M TBAP.
dError limits: 0.05 eV.
Electrochemical properties
The redox potential of m-HRD-1 was evaluated by using cyclic and differential pulse voltammetric techniques in DMF solvent with 0.1 M tetrabutyl ammonium perchlorate as a supporting electrolyte and their data was compared to the standard sensitizer HRD-1 in Table 1 (Figure 3). From the table it is clear that m-HRD-1 undergoes one electron reversible oxidation at 0.67 V vs. SCE. The oxidation process can be readily assigned to the Ru (II)/Ru (III) redox couple. It also undergoes two reductions at -0.95, corresponding to the reduction of ancillary bipyridine ligands. The excited oxidation potential of m-HRD-1 is 1.27 V, which is above the conduction band of TiO2 (41). To identify the electronic distribution of the m-HRD-1 sensitizer, we performed DFT calculations of its electronic ground state using mPW1PW91 method for the geometry optimization with LANL2DZ basis function on Ru and 6-31g (d) basis function on C, H, N, O and S.It can be seen from Figure 4, HOMO, HOMO-1, HOMO-2, HOMO-3 & HOMO-4 are the electrons delocalized over the Ru (II) metal and -NCS ligand. The LUMO, LUMO+1, LUMO+2, LUMO+3 & LUMO+4 are π* orbitals delocalized over the bipyridine carboxylic acid ligand facilitating electron injection from the excited state of the m-HRD-1 sensitizer to the conduction band of TiO2. These results are in line with other ruthenium (II) polypyridyl complexes reported in the literature.44,45
Photovoltaic properties
The performance of the DSSCs was evaluated based on their steady state current- voltage characteristics. Figure 5 depicts the photocurrent density versus photovoltage (J-V) curves of the DSSCs based on standard N719 and m-HRD-1 sensitizers. The photovoltaic parameters including the short circuit current density (Jsc), open circuit voltage (Voc), fill factor (FF) and the power conversion efficiency (PCE) corresponding to the DSSCs are summarized in Table 2. It can be seen that DSSC based on m-HRD-1 dye shows higher photovoltaic performance than the standard N719 dye device. The observed power conversion efficiency is found to be 6.10% under 1.0 sun irradiation (JSC = 18.15 mA/cm2, VOC = 705 mV, ff = 0.48) using m-HRD-1. Whereas, the device based on N719 sensitizer [Jsc = 14.07 mA cm-2, Voc = 668 mV, and ff = 0.49] shows a photovoltaic conversion efficiency of 4.70%. The m-HRD-1 dye based device shows 20% increase in short circuit current due to the better anchoring of dye molecules and thus an increase in overall conversion efficiency.
|
Jsc |
Voc |
FF |
Efficiency |
|
|
Sensitiser |
[mA/cm2] |
[mV] |
[%] |
[%] |
|
N719 |
14.07 |
668 |
49 |
4.7 |
|
m-HRD-1 |
18.15 |
705 |
48 |
6.1 |
Table 2 Photovoltaic parameters of the DSSCs based on N719 and m-HRD-1 sensitizersa
aPhotoelectrode: TiO2 (8+4 mm and 0.2826 cm2).
bError limits: JSC:±0.2 mA/cm2, VOC =±30 mV, ff =±0.3%.
Thermal studies
We examined the thermal stability of the new ruthenium (II) polypyridyl sensitizer and compared it to the thermal stability the standard sensitizer N719, using thermo gravimetric analysis. Figure 6 shows the thermal behavior of m-HRD-1, clearly indicating that the sensitizer m-HRD-1 is stable up to 270 °C. The initial weight loss between 250 to 290 °C is attributed to the removal of the carboxyl group. In contrast, the standard sensitizer N719 is stable up to 200 °C.
In conclusion, we designed and synthesized a new heteroleptic Ruthenium (II) polypyridyl complex having an extended p-conjugation. The complex m-HRD-1 was characterized by various spectroscopic techniques. Both elemental analysis and ESI-MS confirmed the presence of one TBA molecule in its molecular structure. MLCT band of m-HRD-1 was observed at 538 nm in DMF solvent. The emission spectra of m-HRD-1 quenched when adsorbed on TiO2 film as a consequence of electron transfer. Finally, we tested the performance of m-HRD-1 in dye-sensitized solar cells using I-/I3- redox couple and compared to that of the standard sensitizer N719. Thermal studies of m-HRD-1 complex suggested that it was stable up to 270 °C, which was better thermal stable than standard N719 sensitizer.
The authors are thankful to DST (India)-EPSRC (UK) ('APEX-II') programme and the EPSRC Supergen programme for financial support of this work.
The author declares there is no conflict of interest.

©2017 Jella, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.