MOJ
eISSN: 2577-8374


Gonorrhea is the second most prevalent sexually transmitted infection globally. Neisseria gonorrhoeae, the etiological agent of gonorrhea, is evolving into a superbug and may become untreatable due to its resistance to almost all the antibiotics available. There is a critical need for the development of alternative therapeutics. This pilot study aimed to investigate the potential of an innovative non-antibiotic approach, antimicrobial blue light (aBL), as an alternative therapeutic for gonococcal infections. We studied one ATCC strain (ATCC 700825) and one multidrug-resistant clinical strain of N. gonorrhoeae. The results demonstrated that both the strains are highly susceptible to aBL at 405nm. In planktonic suspensions, an exposure of 45 J/cm2 aBL reduced the survival fraction of colony-forming units (CFU) by 7.16-log10 for ATCC 700825 and 2.48-log10 for the clinical strain. When the aBL exposure was further increased to 54 J/cm2, a complete eradication of CFU (over 8-log10 CFU reduction) was achieved for ATCC 700825 and a reduction of 5.43-log10 CFU was obtained for the clinical strain. In addition, we observed that singlet oxygen plays a vital role in the antimicrobial effect of aBL on N. gonorrhoeae. In conclusion, the results of this pilot study suggest that aBL is a promising approach to combat gonococcal infections. Further studies are warranted in the analysis of the endogenous photosensitizers in N. gonorrhoeae cells, evaluation of the aBL efficacy against gonococcal infections in animal models, and investigation of the mechanism of action of aBL.
Keywords: Antimicrobial blue light; Non-antibiotic approach; Gonorrhea; Neisseria gonorrhoeae; Sexually transmitted infection; Antibiotic resistance
aBL, antimicrobial blue light; ATCC, American type culture collection; CDC, centers for disease control and prevention; CFU, colony-forming unit; N. gonorrhoeae, Neisseria Gonorrhoeae; PBS, phosphate-buffered saline; ROS, reactive oxygen species; SOD, superoxide dismutase
Gonorrhea is the second most prevalent sexually transmitted infection globally.1 Despite the public health efforts to control gonorrhea for 70 years, these infections remain a significant public health concern. In 2015, a total of 395, 216 cases of gonorrhea were reported in the United States.2 World widely, 106.1 million people are affected by gonococcal infections annually.3 If gonococcal infections are not appropriately treated, they can result in severe complications and sequelae such as salpingitis and pelvic inflammatory disease, which may lead to sterility and/or ectopic pregnancy. In addition, epidemiologic and biologic studies have provided evidence that the failure to curb the transmission of gonorrhea facilitates the transmission of HIV infection.4 Repeated infections are common and no state of protective immunity appears to develop as consequence of infection. Since there is no gonococcal vaccine, treatment of gonorrhea relies especially on antibiotics. However, Neisseria gonorrhoeae, the etiological agent of gonorrhea, is evolving into a superbug and may become untreatable due to its resistance to almost all the antibiotics previously and currently widely used (e.g., sulfonamides, penicillins, earlier cephalosporins, tetracyclines, macrolides, and fluoroquinolones).5 As such, the Centers for Disease Control and Prevention (CDC) has declared multidrug-resistant N. gonorrhoeae as an urgent threat.6,7 There is consequently a critical need for the development of alternative therapeutics to combat gonococcal infections in high risk communities.8 As the extensive use of antibiotics is the crucial factor leading to multidrug-resistance, non-antibiotic strategy is compelling.9
Antimicrobial blue light (aBL) in the spectrum of 400-470 nm is an innovative non-antibiotic approach that has demonstrated prominent antimicrobial effects without the involvement of exogenous photosensitizers.10 Though the mechanism of action of aBL is still not fully understood, a common hypothesis is that aBL excites the endogenous photosensitizing chromophores (iron-free porphyrins or/and flavins) in microbial cells, and subsequently leads to the production of cytotoxic reactive oxygen species (ROS).10,11 It is envisioned that microbes are less able to develop resistance to aBL than to traditional antibiotics, because of the multi-target characteristics of aBL.10 In addition, it is well accepted that aBL is much less detrimental to host cells than germicidal ultraviolet C irradiation.12,13 However, this approach of using aBL has yet to be studied as a therapeutic for gonococcal infections, especially those caused by multidrug-resistant strains. There have been no any published reports to demonstrate aBL therapy for gonococcal infections.
gonorrhoeae is obligately aerobic. However, many studies have reported that, unlike most other microbes, this bacterium lacks of superoxide dismutases (SODs), which are the major antioxidant defense systems.14 Does it imply N. gonorrhoeae is even more susceptible to aBL inactivation than most other microbes? To answer this question, we carried out a pilot study to evaluate the efficacy of aBL for inactivating N. gonorrhoeae.
We studied one ATCC strain (ATCC 700825) and one multidrug-resistant clinical strain. The results demonstrated that both the strains are highly susceptible to aBL in vitro. In planktonic suspensions, an exposure of 45 J/cm2 aBL at 405 nm reduced the survival fraction of bacterial CFU by 7.16-log10 for ATCC 700825 and 2.48-log10 for the clinical strain (Figure 1). When the aBL exposure was further increased to 54 J/cm2, a complete eradication of CFU was achieved for ATCC 700825 (> 8-log10 CFU reduction, data not shown) and a reduction of 5.43-log10 CFU was obtained for the clinical strain (Figure 1).
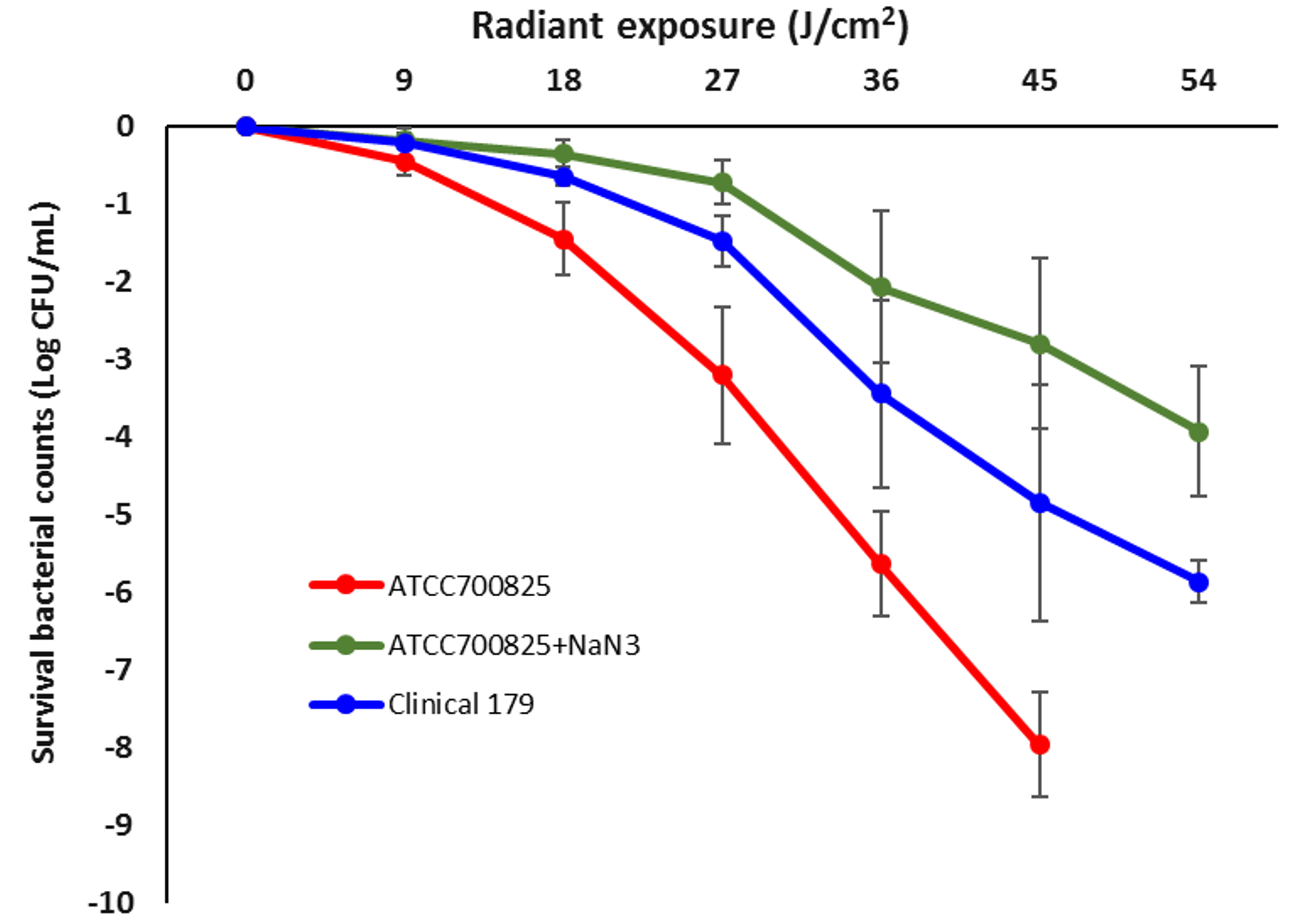
We also investigated whether the effect of aBL is mediated by ROS. By doing so, we added Sodium azide (NaN3), which is known as the specific quencher of singlet oxygen,15 to the suspensions of ATCC 700825 during the illumination of aBL. We observed that the effect of aBL on ATCC 700825 was significantly suppressed due to the addition of NaN3, indicating that singlet oxygen plays a vital role in the antimicrobial effect of aBL on N. gonorrhoeae.
In conclusion, the results of this pilot study suggest that aBL is a promising strategy to combat gonococcal infections. Further studies in our laboratory will be focused on the analysis of the endogenous photosensitizers in N. gonorrhoeae cells, evaluation of the aBL efficacy against gonococcal infections in animal models, and investigation of the mechanism of action of aBL. Due to the anatomical structure of urogenital tract and oropharynx, aBL can be delivered to the infection sites using optical fiber techniques.
This study was supported in part by the National Institute Health (R01AI123312 to TD) and the Department of Defense (FA9550-17-1-0277 to TD). We would like to show our gratitude to Dr. Yonatan H. Grad from Harvard School of Public Health for providing us the clinical strain of N. gonorrhoeae.
Author declares that there is no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.