MOJ
eISSN: 2379-6162


Short Communication Volume 11 Issue 3
Endocrinology and Nutrition Service, Ribera, Hospital POVISA, Spain
Correspondence: C Trigo Barros, Endocrinology and Nutrition Service. Ribera, Hospital POVISA, Spain
Received: June 24, 2023 | Published: August 30, 2023
Citation: Barros CT, Varela RB, González AM, et al. Volanesorsen and familial chylomicronemia syndrome. MOJ Surg. 2023;11(2):114-116. DOI: 10.15406/mojs.2023.11.00235
Objective: To reduce severe hypertriglyceridaemia and episodes of pancreatitis in patients with familial chylomicronemia syndrome (FCHS), in whom the response to diet and triglyceride (TG) lowering treatment has not been sufficient.
Method: A 46-year-old woman diagnosed with genetically confirmed FFCS, with heterozygous presence of two variants and very severe elevation of triglycerides (≥2000 mg/dL), multiple admissions for acute pancreatitis since the age of 19, with associated side effects such as pancreatoprive Diabetes Mellitus with need for insulin and severe hepatic steatosis with grade I fibrosis diagnosed by liver biopsy. Given the intolerance to fibrates and insufficient response to diet and high doses of ω-3 fatty acids, we started treatment with Volanesorsen.
Result: After 6 admissions for acute pancreatitis from January to April 2020, treatment with Volanesorsen was started on 7 August. Platelets at the start of treatment were 283x103/mm3 and triglycerides 1878 mg/dL. Platelet monitoring was performed every 2 weeks and at all times the figure remained >140x103/mm3. The treatment was well tolerated and after three months, the targets for continuing Volanesorsen were reached, reducing TG by more than 25% and reaching 624 mg/dL with platelets in the normal range.
Conclusion: Volanesorsen is indicated as an adjunct to diet in adult patients with genetically confirmed FQS at high risk of pancreatitis, in whom the response to diet and triglyceride-lowering treatment has not been sufficient.
Keywords: hypertriglyceridemia, chylomicronemia, acute pancreatitis
Lipoprotein lipase (LPL) is an enzyme involved in lipoprotein metabolism. It is responsible for hydrolyzing triglycerides circulating in chylomicrons and VLDL (very low density lipoprotein), releasing free fatty acids. To act, it needs apo C-II as a cofactor. LPL or apo C-II deficiency is associated with severe hypertriglyceridemia.1
Familial chylomicronemia is an autosomal recessive syndrome , with severe (1,000-2,000 mg/dL [11.2-22.4 mmol/l]) or very severe (≥ 2,000 mg/dL [22.4 mmol/l]) elevation of TGs, capable of inducing acute pancreatitis and occurring in patients with defects in the genes encoding LPL, or less frequently, other proteins necessary for LPL function such as apoC-II or apoA-V. It is a rare disease whose prevalence ranges between 1-9 per 1,000,000 inhabitants and 25% manifest symptoms during the first year of life.2
The main clinical manifestations are abdominal pain and bloating and slow digestion. They may also present xanthomas (50% of patients), hepatomegaly, splenomegaly and type 3 diabetes. Neurological symptoms (depression and memory loss) and "lipemia retinalis" have also been described.3,4 Acute pancreatitis (AP) is the most serious complication resulting from high triglyceride levels. It is the third cause of AP after alcohol and biliary lithiasis. The prevalence of AP secondary to FQTS is estimated at 67%, mortality ranges between 5-6% and in high-risk patients can reach 30%.2,5
It is not clear that the severity of pancreatitis is directly related to triglyceride levels. Pancreatic lipase activity, efficiency in removing serum fatty acids, and the extent of underlying pancreatic damage are also important. The goal of BP treatment is to maintain TG levels <500 mg/dL (5.6 mmol/L), to avoid necrotizing pancreatitis with multiorgan failure. The long-term goal is to prevent pancreatitis by maintaining TG <1,000 mg/dL (11.2 mmol/L), through strict adherence to hygienic-dietary measures such as a low-fat diet (below 10-15% of daily calories), foods rich in essential fatty acids, fat-soluble vitamins, and avoidance of sugars. The first-line drugs are fibrates and can be associated with ω-3 fatty acids.4
In a high percentage of patients with FQS, conventional treatment fails to control triglyceride levels. It is in these cases that Volanesorsen can be used, provided that at least one of the following circumstances is met: confirmed homozygote or compound heterozygote or double heterozygote for known loss-of-function mutations in the genes causing type 1 (such as LPL, APOC2, GPIHBP1 or LMF1); or demonstrate plasma LPL activity following heparin administration ≤20% of normal.
Volanesorsen is an antisense oligonucleotide designed to inhibit the formation of apoC-III, a protein that regulates TG metabolism and hepatic clearance of chylomicrons and other TG-rich lipoproteins. Selective binding of Volanesorsen to apoC-III messenger ribonucleic acid (mRNA) causes degradation of the mRNA. This binding prevents apoC-III protein translation, which eliminates an inhibitor of TG clearance and activates metabolism by an LPL-independent pathway.
Volanesorsen is presented as a 285 mg solution for injection (1.5 mL volume) in a pre-filled syringe that the patient injects subcutaneously. Initially the dose is weekly and after 3 months the frequency will be reduced to one dose every 2 weeks. However, treatment will be discontinued in patients with a reduction in serum TG concentration <25% or if this concentration does not fall below 22.6 mmol/L, after 3 months of weekly treatment.
After 6 months of treatment, consideration will be given to increasing the frequency of administration to 285 mg per week if the response is insufficient in terms of serum TG reduction, and provided that the platelet count is within the range of normal. If after 9 months of treatment no significant additional TG reduction is achieved, the guideline of 285 mg every 2 weeks will be resumed. The most frequently observed adverse reactions are thrombocytopenia and injection site reaction. Before starting treatment, a platelet count will be taken, with monitoring every 2 weeks.
No dose adjustment is necessary in mild to moderate renal insufficiency and in severe renal insufficiency there are no safety or efficacy data. There are no studies on the use of this drug in patients with hepatic insufficiency but it is not metabolized by the cytochrome P450 pathway. Safety and efficacy in children under 18 years of age have not been established and, although data are limited for those over 65 years of age, no dose adjustment is necessary.4
46-year-old patient diagnosed with severe hypertriglyceridemia in childhood. Father affected but without genetic study. Siblings of the patient are healthy. First admission for AP at the age of 19 years. Since then repeated admissions, initially one per year but since 2016 average of 3 episodes/year. The first assessment by our service is in February 2020, after admission for acute pancreatitis with extraglandular steatonecrosis secondary to severe hypertriglyceridemia with triglycerides of 3573.2 mg/dL. Given the patient's personal history (pancreatoprive Diabetes Mellitus with insulin requirement; severe hepatic steatosis with grade I fibrosis diagnosed by liver biopsy; severe hypertriglyceridemia since childhood-adolescence; ineffectiveness and intolerance of first-line drugs) and 5 admissions for AP from August to December 2019, we requested genetic study detecting in the LPL gene a pathogenic variant c.644 G>A p.(Gly 215Glu) and another probably pathogenic c686A>C p.(TYR233Ser), by massive sequencing with NextSeq sequencerTM (Illumina). The pathogenic variant is described in the clinical databases HGMD (CM900162) and ClinVar (ID: 1522) as a pathogenic variant. It is annotated in the dbSNP database (rs118204057) and in the gnomAD population frequency database (0.06%). The probably pathogenic variant is not described in the literature or in the clinical and population databases consulted. Bioinformatic predictors (SIFT, MutationTaster and Polyphen-2) estimate that the change has a pathogenic effect. At the same position, the variant c.698A>G p.(Tyr233Cys) has also been reported as pathogenic. Based on these data the variant is classified as a Probably Pathogenic Variant.
Given these results, she could be diagnosed with familial hyperchylomicronemia and it was decided to initiate treatment with Volanersorsen in an early care program (PAT), since at that time its use was not approved. This was done with prior authorization from the Servicio Galego de Saúde (SERGAS), the Pharmacy Commission and with the patient's own acceptance by signing the informed consent form.
On August 7, 2020 he started treatment with Volanesorsen, after analytical study (normal renal function; no proteinuria; elevated liver transaminases; normal total, direct and indirect bilirubin; normal blood count). Platelets at the beginning of treatment were 283x10 /mm33 and triglycerides 1878 mg/dL. The initial dose of Volanesorsen is 285 mg, 1.5 mL once a week, with good tolerance to the drug, good management by the patient and with evident clinical improvement, with resolution of the chronic epigastralgia that she presented after ingestion, with tolerance of foods that she withdrew from her diet for this reason (raw vegetables, fatty foods), disappearance of abdominal bloating, heartburn and flatulence. Platelet monitoring was performed every 2 weeks and at all times the figure remained >140x10 /mm33, except in January 2022 when it dropped to 128x10 /mm33 with recovery of figures at one week. The weekly dose was maintained for 3 months and then 285 mg every 2 weeks. From the beginning of treatment until May 2023, she had only one episode of mild acute pancreatitis, which resolved within 48 hours. As an adverse effect, she did present plateletopenia with the need to withdraw Volanesorsen until values above 100x10 /mm33. In March 2023 in analytical control she presented platelets of 88x10 /mm33, so we withdrew treatment, with weekly analytical controls, until reaching 120x10 /mm33 in April 2023, so she resumed Volanesorsen 280 mg every 15 days.
During these weeks without treatment, he has not presented acute pancreatitis or complications derived from plateletopenia such as bleeding.
After starting Volanesorsen, the patient's quality of life has improved considerably, with total resolution of the digestive symptoms she presented and absolute resolution of the work-related problems related to the multiple annual admissions. Her glycemic profile improved, requiring fewer units of insulin. Prior to starting treatment she was on insulin Glargine 32 IU per day with estimated mean blood glucose of 140 mg/dL and A1C of 7%, with current requirements of 10 IU per day, A1C of 5.9% and estimated mean blood glucose of 122.6 mg/dL. Hepatic transaminases normalized after initiation of treatment.
Triglyceride levels improved considerably, reaching a minimum value of 624 mg/dL three months after starting Volanesorsen, with occasional increases of up to 1878 mg/dL. We associate this fact to the fact that it was not used together with omacor (ω-3 fatty acids), due to problems in the dispensing of the latter; however, no new episodes of acute pancreatitis have occurred after the start of treatment despite these punctual elevations of triglycerides (Figure 1). At no time did he present proteinuria, impaired renal or hepatic function and platelets recovered without treatment or complications after temporary withdrawal of the drug (Figure 2 & 3).
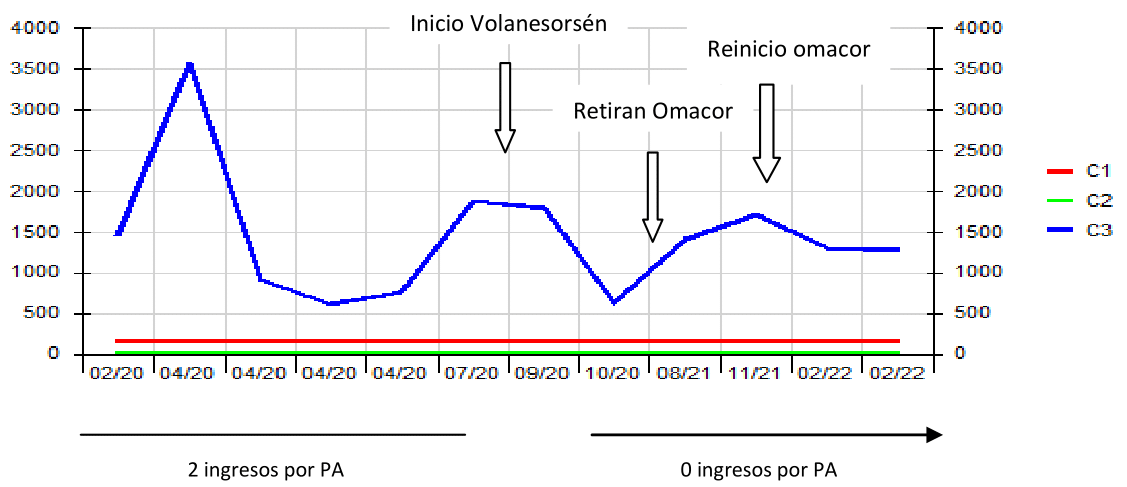
Figure 1 Evolution of TG levels prior to starting Volanesorsén and relationship with AP episodes. In 2020, TG levels were above 1000 mg/dL except during episodes of BP, which improved after fasting. After starting Volanesorsen, basal TG levels decrease and the patient does not have new episodes of BP. TG levels are lower when Omacor is associated. GT: triglycerides. PA: acute pancreatitis.
Volanesorsen is a useful drug to prevent episodes of acute pancreatitis in adult patients with genetically confirmed FQS, in whom the response to diet and triglyceride-lowering treatment has not been sufficient, regardless of the decrease in triglyceride figures. A greater decrease in triglyceride levels has been observed when ω-3 fatty acids are associated.
None.
The authors declare no conflicts of interest.

©2023 Barros, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.