MOJ
eISSN: 2379-6162


Research Article Volume 8 Issue 3
1Junior Resident, Department of General Surgery, Institute of Medical Sciences, Banaras Hindu University, India
2MS, Professor, Department of General Surgery, Institute of Medical Sciences, Banaras Hindu University, Varanasi, UP, India
3Research Scholar, Department of Community Medicine, Institute of Medical Sciences, Banaras Hindu University, India
4MS, Associate Professor, Department of General Surgery, Institute of Medical Sciences, Banaras Hindu University, India
Correspondence: Dr. Vivek Srivastava, Department of General Surgery, Institute of Medical Sciences, Banaras Hindu University,Varanasi, 221005, India, Tel 9453792147
Received: June 15, 2020 | Published: July 14, 2020
Citation: Ali A, Ansari MA, Patel S, et al. Urokinase type plasminogen activator expression and its correlation with angiogenesis and lymph node metastasis in breast cancer. MOJ Surg. 2020;8(3):53-59. DOI: 10.15406/mojs.2020.08.00172
Background: Search for prognostic marker in breast cancer is an area of ongoing research. uPA (Urokinase like plasminogen activator) system has been well documented in cancer invasion and metastasis and extensively researched over the years in different cancers. Familial uPAR (urokinase like plasminogen activator receptor) has been shown to promote promotes angiogenesis.
Objective: To detect the expression of uPA in breast cancer patients and its correlation with established prognostic markers and Vascular Endothelial Growth Factor (VEGF) expression.
Methods: Histologically proven cases of early breast cancer were included in the study who had not received any neo-adjuvant therapy. The patients were characterised based on age at diagnosis, menopausal status, parity, TNM stage. Size of tumor, type of cancer, grade of tumor, tumor necrosis, lymph nodes positive (from sections taken from lymph node), lymphovascular invasion (LVI) and immunohistological details such as hormonal status (ER, PR and HER-2/neu). The expression of VEGF and uPA was done with immunohistochemistry and correlation was analysed.
Results: A total 30 histologically proven early breast cancer patients were included in the study with a mean age of 49.53±9.702 years (range 30-65 years). uPA was expressed in 90% of patients with a mean uPA IRS (immunoreactive score) of 3.567±1.85.The mean VEGF immunoreactive score was 4.16. The uPA expression was significantly associated with T-stage (p<0.001), axillary node (p=0.029), grade of tumor (p=0.028) and lymphovascular invasion (p=0.002) respectively. The positive correlation was found between uPA IRS score and larger tumor size (r=0.727, p<0.001) and number of positive lymph nodes (r=0.466, p=0.009). The correlation between uPA IRS with VEGF IRS was also found to be significant positively correlated (r=0.727, p<0.001).
Conclusion: The results support the importance of uPA expression in breast cancer as a potential for prognosis estimation.
Keywords: breast cancer, uPA, VEGF, lymph node metastasis, uPAR
uPA, urokinase like plasminogen activator; uPAR, urokinase like plasminogen activator receptor; VEGF, vascular endothelial growth factor; LVI, lymphovascular invasion; IRS, immunoreactive score; PTEN, phosphatase and tensin homolog; IHC, immunohistochemical; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma
Attributing to the oldest known malignancy, breast carcinoma still accounts for the leading causes of mortality worldwide. Various clinical parameters have been found to play significant role in tumor development and progression.The recognition of the biologic significance of locoregional recurrence has been an indicator of increased risk for distant disease and nodal metastasis with better understanding of breast cancer biology and its significant clinical implications. Among various known pathways, various proteases have established their role in breast cancer biology. uPA system has been well documented in cancer invasion and metastasis and extensively researched over the years. Familial uPAR (urokinase like plasminogen activator receptor) represses phosphatase and tensin homolog (PTEN) thereby promotes angiogenesis.1 Components of uPA systems increase cell adhesion & migration during metastatic spread of the tumor cells.2 Axillary lymph node status is accepted as the single most powerful prognostic factor. It reveals that the malignancy has gained the ability of systemic spread and the risk of distant metastasis.3 Early prediction of this lymph node metastasis with its proper management leads to better outcomes. Novel markers like uPA pathway have been found to play a role in assessment of extent of neovascularization, lymphovascular invasion, and lymph node metastasis. The present study was done with the aim to detect the expression of uPA in breast cancer patients and its correlation with established prognostic markers and Vascular Endothelial Growth Factor (VEGF) expression.
Histologically proven cases of early breast cancer patients from June 2017 to July 2019 were included in the study. Those giving history of Chemotherapy and or Radiotherapy or history of surgery on breast were excluded. The patients were characterised based on age at diagnosis, menopausal status, parity, TNM stage. Mastectomy specimen were cut into slices at approximately 0.5 cm and the specimen were kept in 10% buffered formalin for 18-24 hours for proper fixation, then the specimen were grossed by trained pathologist to obtain representative tissue sections which were processed and evaluated under light microscopy for evaluation of histopathological details such as: size of tumor, type of cancer, grade of tumor, tumor necrosis, lymph nodes positive (from sections taken from lymph node), lymphovascular invasion (LVI) and immunohistological details such as hormonal status (ER, PR and HER-2/neu), expression of VEGF (vascular endothelial growth factor) and uPA (urokinase like plasminogen activator). Immunohistochemical (IHC) assessment of tumour for uPA was done using unconjugated rabbit anti human uPA polyclonal IgG antibody with immunogen KLH conjugated synthetic peptide derived between 371-422 amino acids of human uPA (dilution 1:400). Primary antibody was obtained from biorbyt Ltd (Cowley Road, Cambridge, United Kingdom). The IHC assessment of tumour for angiogenesis was done by expression of VEGF within tumour cells using rabbit polyclonal anti VEGF IgG antibody with immunogen Human recombinant VEGF 165 obtained from Biogenex (Fremont, California). Results of immunohistochemical staining were graded according to both the intensity and percentage of positively stained tumor cells. Staining intensities of uPA and VEGF were scored on a scale of 0 to 3, with 0 indicating no staining, 1 indicating weak staining, 2 indicating moderate staining, and 3 indicating strong staining (Figure 1 & Figure 2). The percentage of positively stained cells was classified into the following four categories: 0=no positive cells, 1≤10%, 2=10-50%, 3 (51%–80%) and 4 (>80 %) positive cells. In case of discrepancy between duplicate cores, higher score of 2 tissue cores was used as the final score. Level of staining was analysed as immunoreactive score (IRS) Table 1. The data were analyzed using SPSS 16.0 software (SPSS, Inc., Chicago, IL). The mean difference between two groups was calculated by student t test. Categorical variables were evaluated by the two-tailed Chi-square test or two-tailed Fisher’s exact test. The Spearman’s correlation coefficient was used to correlate uPA IRS score with age, tumor size, number of positive lymph nodes and VEGF IRS score. P<0.05 was considered statistically significant.
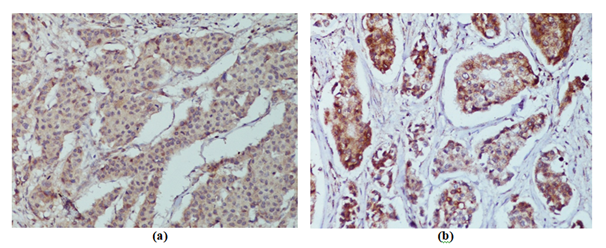
Figure 1 Representative images of uPA, as determined by immunohistochemical staining (x400). uPA exhibited of cytoplasmic immunoreactivity: a) Weak b) Moderate.
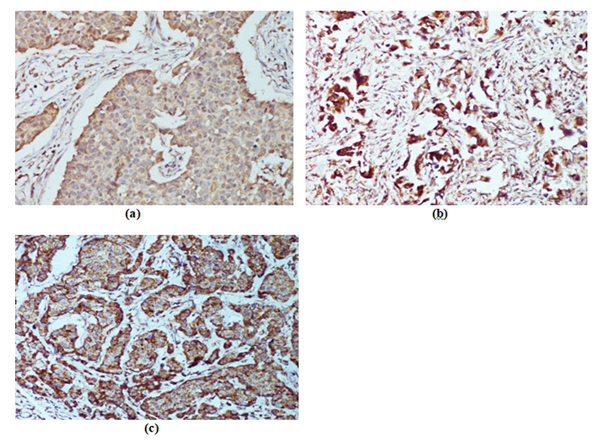
Figure 2 Representative images of VEGF, as determined by immunohistochemical staining (x400). VEGF exhibited variable levels of cytoplasmic immunoreactivity: a) Weak b) Moderate, c) Strong.
|
A (Percentage of positive cells) |
B (Intensity of staining) |
IRS SCORE (AXB) |
|
0= no positive cells |
0= no colour |
0-1 = negative |
|
1=<10 % of positive cells |
1= mild reaction |
2-3= mild |
|
2=10-50 % positive cells |
2=moderate reaction |
4-8=moderate |
|
3= 51-80 % positive cells |
3=intense reaction |
9-12= strongly positive |
|
4=>80 % positive cells |
||
|
Final IRS Score (AXB):0-12 |
||
Table 1 Immunoreactive score (IRS)4
A total 30 histologically proven early breast cancer patients were included in the study with a mean age of 49.53±9.702 years (range 30-65 years). The mean duration of illness was 10.84 months and majority of patients (53.3 %) presented after 6 months of onset of symptoms. Majority of patients (70%) were post-menopausal and rest were pre-menopausal (30%). Lump was present in all patients at the time of presentation. Two patients (6.7%) had history of axillary lump; 2 (6.7%) had history of pain in breast and 2 (6.7%) patients had associated nipple discharge. Parity as a factor influencing estrogen exposure was recorded. Majority (60%) patients had more than 3 children followed by 26.7% (n=8) had only two children and 2 (6.7%) had only one children. Nulliparous women presenting with carcinoma were 2 in number (6.7%). Past history of benign breast disease was present in 3 (10%) of patients which required medical treatment and history of breast feeding was present in 28 (93.3%) of patients. The clinical and pathological characteristics of patients are presented in Table 2.
|
Characteristics |
|
|
Age (years), Mean±SD |
49.53±9.702 |
|
Menstrual status |
|
|
Pre- menopausal |
9 (30.0) |
|
Post Memopausal |
21 (70.0) |
|
Nipple areola complex |
|
|
Normal |
18 (60.0) |
|
Retracted |
12 (40.0) |
|
Size of tumour (cm) |
|
|
<2 |
5 (16.7) |
|
02-May |
23 (76.7) |
|
>5 |
2 (6.7) |
|
Clinical Axillary node |
|
|
N0 |
9 (30.0) |
|
N1 |
21 (70.0) |
|
Histological Type |
|
|
Ductal Carcinoma |
26 (86.7) |
|
Lobular Carcinoma |
1 (3.3) |
|
Adenocarcinoma |
1 (3.3) |
|
Medullary |
1 (3.3) |
|
Metaplastic |
1 (3.3) |
|
Grade of tumour |
|
|
I (Low) |
5 (16.7) |
|
II (Intermediate) |
14 (46.7) |
|
III (High) |
11 (36.7) |
|
Positive Lymph nodes |
|
|
Lymph node metastasis |
14 (46.7) |
|
No Lymph nodal metastasis |
16 (53.3) |
|
Lymphovascular invasion |
|
|
Absent |
20 (66.7) |
|
Present |
10 (33.3) |
|
Molecular subtype |
|
|
ER+/PR+/HER2NEU-(Luminal Type A) |
10 (33.33) |
|
ER+/PR+/HER2NEU+(Luminal Type B ) |
8 (22.22) |
|
ER-/PR-/HER2NEU+(HER+) |
5 (16.67) |
|
ER-/PR-/HER2NEU-(TNBC) |
7 (23.33) |
|
Clinical Staging |
|
|
Stage I |
1(3.3) |
|
Stage II |
27(90.0) |
|
Stage III |
2(6.7) |
|
Pathological staging |
|
|
Stage I |
1(3.3) |
|
Stage II |
22(73.33) |
|
Stage III |
7(23.33) |
|
Clinical Prognostic stage (TNM+GRADE+ER/PR) |
|
|
Stage I |
8(26.7) |
|
Stage II |
16 (53.3) |
|
Stage III |
6 (20.0) |
Table 2 Demographic, clinical and pathological characteristics of patients
The mean tumor size was 3.93±1.33 cm at time of presentation and majority (76.7%) of patients were having tumor size ranging from 2-5 cm. Clinically palpable lymph nodes were present in 21 (70%) patients and they had ipsilateral axillary lymphadenopathy, non matted and not fixed nodes. Ductal carcinoma was the most common cancer type (86.7%) and other types were lobular carcinoma (3.3%), adenocarcinoma (3.3%), medullary variant (3.3%) and metaplastic carcinoma (3.3%). uPA was expressed in 90% of patients with a mean uPA immunoreactive score (IRS) was 3.567±1.85. uPA was moderately positive in 9 (30%) patients, mildly positive in 18 (60%) patients and only 3 (10%) was negatively expressed in uPA. The mean VEGF immunoreactive score was 4.16 and IRS positive VEGF expression was seen in 80% (n=24) of patients out of which majority had moderately positivity (53.3%) followed by 16.67% mildly positive and 10% had strongly positive. The association between uPA and clinic-pathological prognostic factors are shown in Table 3. The uPA expression was significantly associated with T-stage (p<0.001), axillary node (p=0.029), grade of tumor (p=0.028) and lymphovascular invasion (p=0.002) respectively but not with clinical stage, pathological stage, lymph node positivity or molecular subtypes. The Spearman’s correlation coefficient for uPA IRS score with age, tumor size, number of positive lymph nodes and VEGF IRS score are shown in Table 4. No significant correlation was observed between uPA IRS score and increasing age of patients (r=0.292, p=0.118). The positive correlation was found between uPA IRS score and larger tumor size (r=0.727, p<0.001). Number of positive lymph nodes were positively correlated with uPA IRS score (r=0.466, p=0.009) and patients with increase number of positive lymph nodes had highest uPA IRS score. All the cases showing negative staining to uPA also exhibited negative staining to VEGF. Further, 21 out of 27 patients were mild or moderate staining to uPA showed similar staining to VEGF. However, three patients having strong VEGF IRS had moderate uPA IRS. The correlation between uPA IRS with VEGF IRS was found to be significant positively correlated (r=0.727, p<0.001) Figure 3.
|
uPA IRS |
p-value |
|||
|
Parameters |
Negative |
Mild |
Moderate |
|
|
N (%) |
N (%) |
N (%) |
||
|
T-stage |
||||
|
T1 |
2(66.7) |
0(0) |
1(11.1) |
<0.001 |
|
T2 |
1(33.3) |
16(88.9) |
7(77.8) |
|
|
T3 |
0(0) |
2(11.1) |
1(11.1) |
|
|
Axillary node |
||||
|
No node |
2(66.7) |
4(22.2) |
3(33.3) |
0.029 |
|
Node present |
1 (33.3) |
14 (77.8) |
6(66.7) |
|
|
Clinical stage |
||||
|
I |
1 (33.3) |
0 (0) |
0 (0) |
0.225 |
|
II |
2 (66.7) |
17(94.4) |
8 (88.9) |
|
|
III |
0 (0) |
1 (5.6) |
1 (11.1) |
|
|
Pathological stage |
||||
|
Stage 1 |
1(33.3) |
0(0) |
0(0) |
0.405 |
|
Stage 2 |
2(66.7) |
16(88.89) |
4(44.44) |
|
|
Stage 3 |
0(0) |
2(11.11) |
5 (55.56) |
|
|
Grade of tumour |
||||
|
I (Low) |
2 (66.7) |
1 (5.6) |
2 (22.2) |
0.028 |
|
II (Intermediate) |
0 |
8 (44.4) |
6 (66.7) |
|
|
III (High) |
1 (33.3) |
9 (50.0) |
1 (11.1) |
|
|
Lymphovascular invasion |
||||
|
Absent |
3 (100) |
15 (83.3) |
2 (22.2) |
0.002 |
|
Present |
0 |
3 (16.7) |
7 (77.8) |
|
|
Positive Lymph Node |
||||
|
Present |
1 (33.3) |
6 (33.3) |
7 (77.8) |
0.082 |
|
Absent |
2 (66.7) |
12 (66.7) |
2 (22.2) |
|
|
Molecular subtypes |
||||
|
Luminal A |
2 (66.67) |
7 (38.88) |
1 (11.11) |
0.596 |
|
Luminal B |
1 (33.33) |
4 (22.22) |
3 (33.33) |
|
|
HER + |
0 |
3 (16.67) |
2 (22.22) |
|
|
TNBC |
0 |
4 (22.22) |
3 (33.33) |
|
Table 3 Association between uPA IRS and prognostic factors
|
Variables |
uPA IRS |
|
|
r-value |
p-value |
|
|
Age (years) |
0.292 |
0.118 |
|
Tumor size (cm) |
0.756 |
<0.001 |
|
Number of Positive Lymph nodes |
0.466 |
0.009 |
|
VEGF IRS score |
0.727 |
<0.001 |
Table 4 Spearman’s correlation coefficient for uPA IRS score with age, tumor size, number of positive lymph nodes and VEGF IRS score
Numerous studies have investigated the clinical significance of tissue expression in uPA/PAI-1 complex in cancer of various organs, such as: stomach,5 colon and rectum,6 ovary,7 endometrium8 and lungs9,10 showing its prognostic significance. In our study, the mean age of presentation was 49.53±9.702 years with majority of patients in 5th decade of life. Similar result was observed by Raina et al.,11 in which the mean age of presentation was less than 50 years which is lower than that in developed countries. Another study by Hadi et al.,12 carried on 654 patients treated over a span of 18 years from 2000 to 2017, the age of presentation ranged from 24-91 years with the mean age of 48 years. There was a lower occurrence in both lower and higher age groups. The study also showed a rise of mean age of presentation from early forties in 2000 to the late fifties in 2016/17. In the present study, all patients presented with history of lump in breast with 2 patients (6.7%) each having history of lump axilla and nipple discharge. According to duration of symptoms; 53% of patients presented with total duration exceeding 6 months. The mean duration of presentation was 10.847 months. Koo et al.,13 conducted a study in South East Asian countries providing detailed evidence about the symptom signature of breast cancer. Out of 56 presenting symptoms breast lump was the commonest, accounting for 83% followed by nipple abnormalities (7%) and pain in breast (6%). In their study women presenting with ‘lump only’ had the shorter median interval of presentation (2 months) compared to those with ‘non-breast lump’ symptoms (3 months). Various older studies have shown parity as a protective factor for breast cancer. However modern studies have revealed that parous women had lower incidence of ER positive breast cancer compared to nulliparous breast cancer patients, however no association was observed for ER negative disease.14 Sixty percent of women in our study (n=18) were multiparous. Nulliparous women presenting with carcinoma were 2 in number (6.7%) and had more severe histological grade. The sample included 70% of patients who were post-menopausal. Past history of benign disease was present in 10% of the women requiring medical treatment. Majority of the patients (93%) had history of breast feeding. Most of studies have shown parity and breastfeeding as preventive factors in development of breast cancer although the number of breastfed children, the period of breastfeeding, age at first lactation, and period of amenorrhea during lactation are the parameters to be taken into account.15,16
In our study, majority of cases (86.7%) who presented were histologically ductal carcinoma with 3.3% of cases as lobular carcinoma and another 3.3% as medullary variant and other as adenocarcinoma and metaplastic varieties. According to AJCC, incidence of invasive ductal carcinoma (IDC) is 50-75% and Invasive lobular carcinoma (ILC) is 5-15% and breast carcinomas with medullary features as less than 1%. We found that the uPA expression was significantly associated with T-stage (p<0.001), axillary node (p=0.029), grade of tumor (p=0.028) and lymphovascular invasion (p=0.002) respectively and no significant association was observed between clinical stage, pathological stage, positive lymph node and molecular subtype. The Spearman’s correlation coefficient for uPA IRS score with age, tumor size, number of positive lymph nodes and VEGF IRS score showed no significant correlation between uPA IRS score and increasing age (r=0.292, p=0.118). The positive correlation was found between uPA IRS score and larger tumor size (r=0.727, p<0.001), number of positive lymph nodes (r=0.466, p=0.009) and VEGF IRS (r=0.727, p<0.001). In a study by Andres et al.,17 conducted a retrospective study on 226 cases of breast cancer and evaluated level of uPA expression and compared it with patient characteristics, histopathological features, and clinical outcome. Linear regression of the scatter plots revealed no significant correlation of age with uPA (p=0.70, n=221). Foekens et al.,18 studied 2780 patients with primary invasive breast cancer were evaluated the prognostic importance of the uPA system was correlated with age, menopausal status, tumor size and grade, lymph node status, in the same study Spearman’s correlation of level of uPA did not significantly relate with age, menopausal status, or lymph node status. Well-differentiated and T3/4 tumors had the lowest uPA levels, whereas moderately differentiated and T2 tumors had the highest levels. The present study also showed that T2 tumours had maximum expression of uPA among all T stages Figure 4.
Kim et al.,19 established in their study conducted over 214 patients (2006 to 2010) that high uPA expression was detected in higher percentage of patients with lymph node metastasis (56.3%) than in patients without lymph node metastasis (33.6%) (p=0.001).The findings of correlation of uPA expression with p stage, LVI and positive lymph nodes is suggestive of similar finding in previous study. Duffy et al.,20 have established uPA as prognostic biomarker in lymph node-negative breast cancer. Harbeck et al.,21 conducted a multicenteric prospective randomized therapy trial in node-negative breast cancer cohort, results from the study showed that node-negative breast cancer patients with low levels of uPA in their primary tumor had very good prognosis, and could be spared from the burden of adjuvant chemotherapy whereas node-negative patients with high uPA were at substantially increased risk of disease recurrence. Choi et al.,22 has shown that intratumoral microvessel density is an important prognostic marker of survival in breast cancer and for prediction of the likelihood of systemic metastases. Consistent with their role in cancer dissemination, high levels of uPA in multiple cancer types correlate with dismal prognosis. Correlation of uPA expression with VEGF was established by Stepanova et al.,23 who indicated uPA-dependent de-repression of VEGFR1 and VEGFR2 gene transcription by which uPA mediates the pro-angiogenic effects of VEGF and identifies a potential new target for control of pathologic angiogenesis. Huber et al.,24 studied TNBC cell lines and established direct tumour promoting interaction of uPAR and uPA and also found that these interactions were significantly reduced when uPAR was down regulated. This study pointed toward a novel therapeutic target in TNBC patients in whom targeted therapy is limited.
Antiangiogenic therapy is a hot cake in modern oncology because most of the angiogenic ligands and receptors are functionally active in tumour mass progression and can share some combinative actions with lymphatic vessels growth. Consequently, the rationale for anti-angiogenic therapy can favour the cessation of lymphatic vessels development, which in turn potentially hampers the metastatic budding of the tumor cells.25 Angiogenesis is seen to be dependent on uPA expression in breast cancer hence anti angiogenic therapy could be a potential tool in treatment and prevention of locoregional spread and distant metastasis in patients having higher uPA expression. uPA expression has shown positive correlation with Lymph node spread in various studies including the present study. Lymph node metastasis is single most established poor prognostic marker in breast cancer patients. This important tool is missing in node negative breast cancer patients who may be having histologically undetectable nodal metastasis. uPA expression can serve as surrogate marker for potential nodal recurrence in such subset of patients. The limitation of present study is small sample size which is the probable reason for not having significant association and correlation of few of the established prognostic parameters. The positive results of remaining prognostic parameters qualifies the present study to be a stepping stone towards a larger combined retrospective and prospective study to have a better insight Figure 5.
In conclusion of the present study the level of uPA expression correlated with level of VEGF expression and Lymphovascular invasion and number of positive lymph nodes. Angiogenesis and lymphangiogenesis system shows a potential new target for development of anti-cancer strategies thereby opening new horizon for anti cancer targeted therapy. uPA expression also holds a potential for establishing as a single prognostic marker although larger, multicentric prospective study will be needed to establish its role in future.
None.
None of the authors declares conflicts of interest.
None.

©2020 Ali, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.