MOJ
eISSN: 2379-6162


Mini Review Volume 12 Issue 3
Department of Surgery, Hospital Angeles Lomas, México
Correspondence: Alejandro Weber Sánchez, Department of Surgery, Hospital Angeles Lomas, Huixquilucan, México
Received: October 15, 2024 | Published: October 31, 2024
Citation: Weber-Sánchez A, Weber-Alvarez P, Garteiz-Martinez D. Proposal of proximal gastric plication as a simple technique to prevent gastroesophageal reflux disease after sleeve gastrectomy. MOJ Surgery. 2024;12(3):109-111. DOI: 10.15406/mojs.2024.12.00274
Proximal gastric plication is a simple technique that has been employed by the authors to treat gastroesophageal reflux disease (GERD) in patients who have developed symptoms after being subject to sleeve gastrectomy (SG). Because of the favorable results observed in these cases, it is now proposed that this procedure be performed initially, in all patients who undergo sleeve gastrectomy, to avoid de novo GERD. The technique is described in this article.
Keywords: proximal gastric plication, gastroesophageal reflux disease (gerd), bariatric surgery, sleeve gastrectomy (sg)
Contemporary data shows that sleeve gastrectomy (SG) is the most preferred and performed bariatric procedure worldwide because of its effectiveness, low mortality, and reduced side effects when compared to gastric bypass. Nonetheless, de novo or worsening gastroesophageal reflux disease (GERD) is a concern as a possible secondary effect of the procedure. Due to the heterogeneity among studies and controversial results of esophageal function tests, the exact effect of laparoscopic SG on the prevalence of GERD remains unanswered.1,2 Some operative techniques have been suggested to avoid this problem, and other treatment strategies have been designed to manage postoperative GERD. During the past decade, the authors have proposed proximal gastric plication as a simple technique to create an internal valve to treat GERD in patients with previous bariatric surgery and have had good results.3 This report aims to make the proposal that de novo GERD may be prevented by performing the proximal gastric plication in patients who undergo SG for the first time.
Two 12mm trocars are used: one through the umbilicus, and the other over the left midclavicular line for the stapler. Three additional 5mm trocars are then placed, one subxiphoid for the liver retraction with a grasper, another subcostal over the left anterior axillary line, and the last one over the left mid-axillary line for retraction and the 5mm 30º laparoscope changing it between these two trocars as necessary. All patients diagnosed with, or found during surgery to have a hiatal hernia, are first subject to its repair by dissecting the hiatal crura and closing the defect with two or three sutures (Ethibond 2-0, Ethicon J&J®) on the posterior aspect, and when necessary with one or two more sutures on the anterior hiatus.
The SG is then performed by dividing the gastroepiploic omentum from the greater curvature with an ultrasonic scalpel (Sonicision TM Medtronic) starting 6cm from the pyloric vein to the angle of His, until the left crura of the hiatus was observed. The gastro-splenic and gastro-phrenic ligaments are also divided. The fat pad that covers the His angle is excised with the same instrument. A 36Fr bougie is passed to the stomach to construct the gastric tube using five or six cartridges of the Endo GIA 60 tristaple (CovidienTM) to divide the greater curvature. The proximal gastric plication is then performed to create an internal antireflux valve in the upper 3cm on the lateral side of the gastric tube by using a grasper to invaginate 2cm of the gastric wall of the stomach with sero-muscular stitches of a running suture of 2-0, 45cm spiral Monocryl (Stratafix Ethicon ®), and then continue the suture to reinforce the rest of the staple line (Figure 1-3). This invagination reduces the gastric bulb that is frequently observed at the proximal staple line and serves to decrease the gastric lumen at this point; serving as an internal anti-reflux valve.
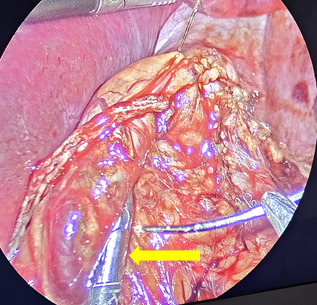
Figure 2 Grasper invaginating 2cm of the upper stapled side of the stomach tube wall to create the antireflux valve with proximal gastric plication.
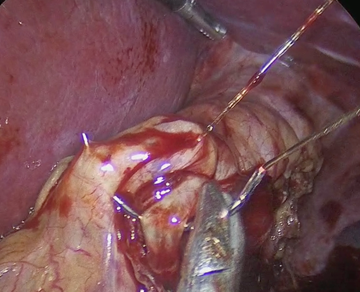
Figure 3 Running suture with 2-0 sero-muscular inverted Stitches to reinforce the rest of the stapled line.
Finally, the new greater curvature is fixed again to the greater omentum with five or six stitches with 2-0 Polyglactin 910 (Vicryl Ethicon ®), and a 10mm drain is left in place (Biovac Biometrics ®) and exteriorized through the incision of the left mid-axillary line after the extraction of the excised stomach through the extended umbilical incision.
Sleeve gastrectomy is the most frequently performed bariatric procedure today because it is efficient and safe.4,5 However, a common long-term complication reported is the increased rate of gastroesophageal reflux disease.6–9 The actual prevalence of GERD after SG is not known and has been informed with a wide range of variability. On the other hand, some studies have shown improvement in GERD after SG.10–14 According to the American Gastroenterological Association clinical practice update on the personalized approach to the evaluation and management of GERD, a plan for the investigation of GERD symptoms must be personalized. Obese patients are not the exception.15 The American Society for Metabolic and Bariatric Surgery considers erosive reflux esophagitis as a relative contraindication for SG.16
Therefore, a preoperative diagnostic work-up should be done in obese patients when designing a surgical bariatric strategy.17 If severe GERD or Barret’s disease is detected, probably Roux-Y gastric bypass should be preferred as the first choice. Different anatomical and pathophysiological mechanisms have been proposed for the development of GERD after SG. Disruption of the oblique fibers of the gastroesophageal sphincter due to stapling too close to the angle of His decreasing lower esophageal sphincter pressure, a dilated upper sleeve, a narrow distal sleeve gastrectomy that increases the intragastric pressure, or the development of gastric emptying or esophageal motility disorders, among others.18–20 Concomitant hiatal hernia should be corrected to preserve the intra-abdominal length of the esophagus and to prevent migration of the gastric tube to the mediastinum.21
Varied approaches have been suggested to deal with GERD after SG, since conservative measures, endoscopic interventions, endoluminal stents, balloon dilations, endoscopic radiofrequency (Stretta), antireflux mucosectomy, or surgical management. But none have shown consistent results.6 Also, to prevent de novo GERD, surgical approaches such as SG plus fundoplication and other changes in surgical technique have been described but adding an anti-reflux procedure when performing SG is a controversial subject, and the procedure has remarkable complications such as gastric perforation.22,23
We have had clinically good results with the proximal gastric plication procedures we performed over the years to correct GERD in patients with different bariatric procedures including SG.3 The internal anti-reflux valve can be seen clearly in the X-ray and endoscopic studies we have performed after the surgery and has been found to persist in patients operated on more than five years after the original procedure (Figure 4).
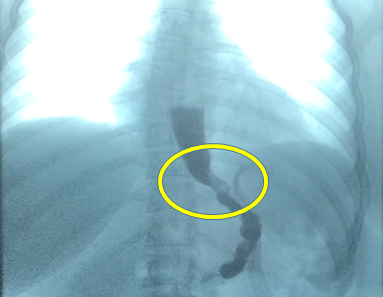
Figure 4 A detailed photo of the internal anti-reflux gastric valve constructed with the proximal gastric plication.
Since this proposal is for all patients who undergo sleeve gastrectomy, no special preoperative studies are required other than those done routinely. Obviously, if a patient has GERD before surgery it is important to perform endoscopy, manometry, or pHmetry in selected cases and in accordance to their reflux symptoms to select the best surgical approach. But because of their risk of developing reflux after SG, we suggest that these patients without GERD undergo the proximal gastric plication, and if they have hiatal hernia it should be corrected. Long-term prospective randomized studies that compare patients with and without gastric plication could give us a better insight of the benefits of this procedure.
Although further studies are required to evaluate the long-term results, our experience suggests that proximal gastric plication is a promising procedure to reduce the incidence of postoperative GERD in sleeve gastrectomy patients.
None.
The authors declare that there are no conflicts of interest.

©2024 Weber-Sánchez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.