MOJ
eISSN: 2379-6162


Case Report Volume 10 Issue 1
1Medical Doctor and Surgeon, Universidad Nacional de Colombia, Hospital Universitario Nacional de Colombia, Colombia
2Resident of General Surgery, Universidad Nacional de Colombia, Hospital Universitario Nacional de Colombia, Colombia
3General Practitioner, Universidad CES, Colombia
4Medical Specialist in General Surgery, Universidad Nacional de Colombia, Colombia
5Medical Specialist in Thoracic Surgery, Universidad Nacional de Colombia, Colombia
6Pathology Specialist. Professor, Pathology Deparment, Universidad Nacional de Colombia, Hospital Universitario Nacional de Colombia, Colombia
Correspondence: Andrés Ricardo Latorre-Rodríguez MD, Medical Doctor and Surgeon, Universidad Nacional de Colombia, Hospital Universitario Nacional de Colombia, Carrera 45 N° 26-85, Medicine School, Universidad Nacional de Colombia, Bogotá DC, Colombia, Tel +57 3204264529
Received: July 15, 2022 | Published: July 27, 2022
Citation: Latorre-Rodríguez AR, González-Fajardo J, Giraldo-Restrepo JP, et al. Primary synovial sarcoma of the lung: Case report and review of the literature. MOJ Surg. 2022;10(1):9-11. DOI: 10.15406/mojs.2022.10.00194
Primary synovial sarcoma of the lung is a rare pathology, representing around 0.5% of malignant lung neoplasms. Clinically, it is manifested by pleuritic chest pain, cough, hemoptysis, dyspnea, and weight loss. Diagnosis requires a good clinical history, imaging, and clinical and immunohistochemical studies. Its treatment is not fully established. However, complete surgical resection associated with chemotherapy has shown improvements in survival. This report presents the case of a 26-year-old male patient with dyspnea, chest pain, cough, and hemoptysis.
Subsequently, a mass in the left hemithorax was evidenced using radiological studies, and surgical resection was performed. The anatomopathological and immunohistochemical findings suggest synovial sarcoma, for which a co-adjuvant chemotherapeutic scheme was started; the patient had an operative site infection during hospitalization and required intravenous antibiotic therapy, with subsequent favorable clinical evolution.
Keywords: synovial sarcoma, pulmonary neoplasms, thorax, immunohistochemistry, diagnosis
Primary synovial sarcoma of the lung is an extremely rare entity, representing less than 0.5% of pulmonary neoplasms1 and 10% of soft tissue sarcomas.2 Being a malignant tumor, with a 5-year survival that is significantly worse than soft tissue sarcomas of the extremities (35% vs. 71%)3; It equally affects both sexes and hemithorax.4 The diagnosis is based on clinical findings such as the presence of chest pain (24-80%), dyspnea (8-36%), cough (8-33%), and hemoptysis (20-25%).5,6
Alternatively, the presence of complete opacification of the hemithorax is generally documented. The tomography reveals a mass with well-defined homogeneous or heterogeneous enhancement, which contains areas of attenuation compatible with necrosis or hemorrhage, pleural effusion suggestive of hemothorax can be seen, and ground glass opacities have been reported around the mass, mediastinal lymphadenopathy is rare.5,6
At the histopathological and immunohistochemical level, spindle-shaped mesenchymal tumor cells are found, with variable degrees of epithelial and glandular differentiation,7,8 subdivided into four categories biphasic, monophasic fibrous (spindle cells or spindle cells), monophasic epithelial and poorly differentiated.7 Cytogenetically, the translocation t(X;18) (p11.2;q 11.2) is evidenced, generating the fusion of the SYT gene of chromosome 18 with SSX1 or SSX2 of the X chromosome. Differential diagnosis with metastatic disease from X must be considered for extrapulmonary sarcomas one and pleural neoplasms.8 The treatment is diverse and has not been standardized according to its rarity. However, complete surgical Excision associated with radio/chemotherapy offers the best chances of cure.3,4 We present the clinical case of a young patient with a left lung mass evidenced in tomography, who was taken for resection and lower lobectomy, with histopathological and immunohistochemical findings that showed a synovial sarcoma.
A 26-year-old male patient consults due to a four-month history of occasional pain in the left hemithorax with pleuritic characteristics, exacerbated in the last month, without improvement with conventional analgesics, associated with a dry cough that later evolved into hemoptysis and progressive deterioration of the functional class. In addition, the patient reported an unintentional loss of 10 kg in the last month.
The only relevant medical history was moderate smoking. Physical examination revealed high heart rate (102 bpm), tachypnea (22 bpm) and mild desaturation (FiO2 89%), blood pressure was normal (109/71 mmHg); Auscultation documented hypoventilation of the entire left lung field, with no evidence of palpable lymphadenopathy or enlarged lymph nodes.
The paraclinical tests and laboratory tests showed mild leukocytosis with neutrophilia, serial bacilloscopies were performed with normal results, the chest X-ray showed opacity throughout the left lung field (Figure 1A & 1B), and the high-resolution contrast-enhanced tomography of the chest showed a solid extrapulmonary mass with well-defined borders, with heterogeneous density suggestive of cystic degeneration and/or localized necrosis in the middle and lower third of the left thoracic cavity, measuring 124 x 153 x 167 mm (T x AP x L), and was found in close contact with the pericardium, the chest wall, lymphadenopathy is not defined (Figure 2A–2C).

Figure 1 Chest X-ray. Mass in the left hemithorax displaces the cardio-mediastinum to the right and the left main bronchus superiorly. 1A) PA projection, 1B) lateral projection.
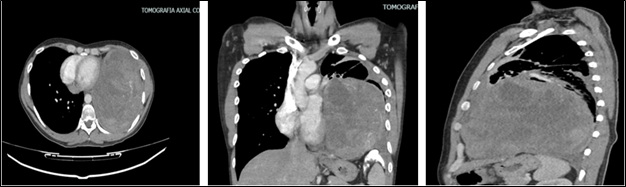
Figure 2 Contrasted computed tomography (CAT) of the chest. Solid extrapulmonary mass that occupies the entire left hemithorax, details of multiple projections.
Tru-cut biopsy was performed; however, the sample was not significant, which is why it was scheduled to perform a left anterolateral thoracotomy with intraoperative findings of a large tumor mass with softened content, surrounded by a thick capsule, which compromises the lower lobe lung parenchyma, with old clots, involving the entire left pleural cavity and extending from the diaphragm to the pericardium, the wall and the parietal pleura (Figure 3A–3C).
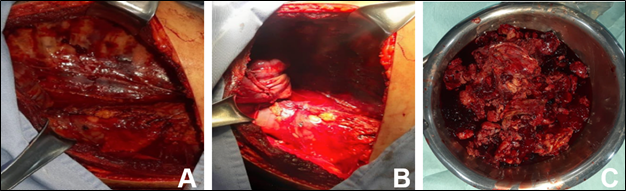
Figure 3 A) Large tumor mass that involves the lung parenchyma of the left lower lobe and occupies most of the ipsilateral hemithorax. B), Excision of the lung mass and resection of the left lower lobe. C) Left lung tumor mass resection product.
Extraction of this was performed, the release of the diaphragmatic portion of the mass with partial resection of the same with posterior repair, it was bluntly separated from the posterior wall, extrapleural dissection, it was resected from the diaphragm, and a partial evacuation of the intraparenchymal injury. There was no evidence of lymph node involvement, nor did it require pericardial resection. Bleeding was estimated at 1500CC, which required a transfusion with units of red blood cells; no other complications were documented.
The pathological study revealed a monomorphic, undifferentiated malignant mesenchymal tumor made up of spindle cells arranged in short bundles, with a mitotic count of 14 mitoses in 10 40x fields; tumor cells showed intense immunoreactivity for TLE-1, BCL-2, Calretinin and WT-1, and other markers practiced (AE1/AE3, CK5/6, EMA, CD99, CD34, AML, S100, and D2-40) were negative, findings compatible with synovial sarcoma, histological grade 3 FNCLCC, without nodal or lymphovascular involvement, and tumor-free section margins (Figure 4 & 5). Therefore, chemotherapy treatment was started co-adjuvant with Doxorubicin and Ifosfamide (MAI).
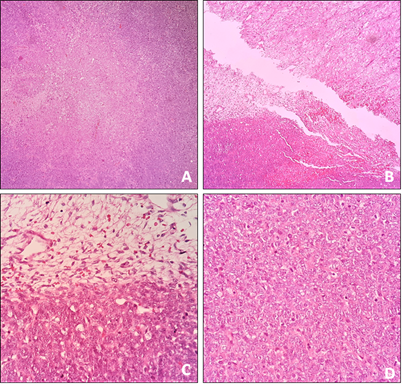
Figure 4 Basic staining study. A) Malignant neoplasm of mesenchymal origin forming sheets of small spindle cells intercepted by delicate fibrovascular septa and focal coagulative necrosis (HE, 4x). B and C) Cellularity is marked, although hypocellular areas with a loose and edematous appearance are also identified (HE, 4-40x). D) The neoplastic cells are round, elongated with scarce cytoplasm, and poorly defined; the nuclei are monotonous with mild pleomorphism, fine chromatin, and frequent mitoses, including atypical forms (HE, 40x).

Figure 5 Immunohistochemical study. Intense and diffuse immunoreactivity in tumor cells for Bcl-2, TLE-1, Calretinin, and WT1 (cytoplasmic) (10-40x).
During hospitalization, the patient presented an infection of the operative site organ-space in the left hemithorax due to Enterococcus faecalis, for which he required antimicrobial treatment with broad-spectrum beta-lactam; given the adequate evolution, the patient was discharged and continued chemotherapy treatment, two months later the patient has not presented additional postoperative complications, and his tolerance to oncological management has been adequate.
Primary synovial sarcoma of the lung is a rare entity representing between 0.1% - 0.5% of all pulmonary neoplasms, unlike synovial sarcomas located in the extremities, which represent more than 90%. This entity typically occurs in adolescents and young adults, like the one described in our clinical case, and generally does not have a good prognosis.5,6 Histologically, it is classified into four categories: biphasic, monophasic fibrous (spindle cells or spindle cells), epithelial monophasic, and poorly differentiated. These tumors can present an aggressive behavior, infiltrating adjacent structures.6 Immunohistochemistry plays an essential role in diagnosis. This tumor frequently shows immunoreactivity for Bcl-2, TLE-1, CD99, CD56, SYT, Calretinin, and variably for cytokeratins. On the other hand, it is usually negative for S-100, SOX10, CD34, desmin, smooth muscle actin, and other vascular tumor markers. Findings were found concerning the clinical case presented (Table 1). Cytogenetic studies can show the t(X;18) translocation (p11.2; q11.2) resulting in the fusion of the SYT gene on chromosome 18 with SSX1 or SSX2 on the X chromosome, being both SS18-SSX1 and SS18-SSX2 more frequent in the monophasic presentation (60-70% and 97%), compared to the biphasic (30-40% and 3% respectively).6 The differential diagnosis involves other types of malignant lung neoplasms such as fibrosarcomas, carcinosarcomas, leiomyosarcomas, mesothelioma, or hemangiopericytomas. In addition, it is always necessary to rule out metastatic lung disease.6
Treatment is not standardized; however, surgical intervention plays a predominant role and is the primary strategy. Despite not having recommendations or expert opinions on optimal surgical treatment, extensive tumor resection is currently performed (it has been found to control local relapse) to achieve R0 margins.4–6 Most cases of operability are treated with lobectomy or pneumonectomy by virtue of tumor size. In some cases, partial or complete lumpectomy combined with removal of mediastinal lymphoid nodules may be appropriate. In advanced stages, with locally invasive, metastatic disease with unresectability criteria, radiotherapy and/or chemotherapy based on Doxorubicin and Ifosfamide is useful.4,6 Some authors consider that extensive pneumonectomy associated with radiation or adjuvant chemotherapy may be the treatment of choice since it ensures maximum resection and improves survival in young patients.6 The prognosis is usually poor, with a 5-year survival rate of 50%. Negative prognostic factors include tumor size, male gender, extensive necrosis, high histologic grade, mitotic rate, neurovascular invasion, and SYT-SSX1 expression.5
Primary synovial sarcoma of the lung is a rare tumor with a poor prognosis that should be suspected in middle-aged patients; its diagnosis requires an adequate clinical history, suggestive imaging findings, and a thorough histopathological and immunohistochemical analysis. In some cases, molecular cytogenetic testing is necessary for the t(X;18) translocation (p11.2; q11.2) to confirm the diagnosis. Treatment involves surgical resection with negative margins associated with chemotherapy or radiotherapy. Given the high rates of recurrence, close monitoring of patients is required.
None.
We declare that there was no conflict of interest in preparing this article. All authors of this document participated in the writing, editing, and general design.

©2022 Latorre-Rodríguez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.