MOJ
eISSN: 2379-6162


Research Article Volume 8 Issue 1
1Kulvinder Kaur Centre For Human Reproduction, India
2Rotunda-A Centre for Human reproduction, India
3Consultant Neurologist, Swami Satyanand Hospital, India
Correspondence: Kulvinder Kochar Kaur, Kulvinder Kaur Centre for Human Reproduction, 721, GTB Nagar, Jalandhar-144001, India, Tel 91-181-4613422, Fax 91-181-4613422
Received: April 26, 2019 | Published: January 27, 2020
Citation: Kaur KK, Allahbadia G, Singh M. Management of azoospermia with special emphasis on microdissection testicular sperm extraction (mTESE) in non obstructive azoospermia prior to IVF/ICSI to optimize sperm retrieval rates, pregnancy, live birth rates–a systematic review. MOJ Surg. 2020;8(1):7-16. DOI: 10.15406/mojs.2020.08.00162
Previously there was no option for infertility due to non obstructive azoospermia (NOA). But with advent of technology NOA has become treatable with the use of testicular sperm extraction and IVF. The most correct approach for sperm retrieval remains microdissection testicular sperm extraction (mTESE). This systematic review discusses and examines the literature in terms of Patient optimization before mTESE, Technique of mTESE, along with post mTESE testicular tissue processing. Preoperative patient medical therapy, data for varicocele repair support increased sperm retrieval, pregnancy and return of sperm in the ejaculate. Post mTESE tissue processing has few studies where comparison has been done, though most studies support the combination of mechanical mincing and use of type 4 collagenase for tissue disintegration along with pentoxifylline in assisting in identification of motile and viable spermatozoa for intractoplamic injection.
Keywords: microdissection testicular sperm extraction(mTESE), NOA, azoospermia, neoadjuvant hormone therapy, testicular tissue processing
NOA, non obstructive azoospermia; mTESE, microdissection testicular sperm extraction; SRR, sperm retrieval rate; SCOS, sertoli cell only syndrome; STs, seminiferous tubules; OA, obstructive azoospermia; KS, klinefelters syndrome; HPA, hypothalamo-pituitary-axis; KS, kallmann syndrome; EDO, ejaculatory duct obstruction; TRUS, transrectal utrsound; AZF, Azoospermia factors; ST, seminiferous tubule; DT, dilated tubules; NDT, non dilated tubules; CC, clomiphene citrate; ART, assisted reproductive technology; PCNA, proliferative cell nuclear antigen.
Of the 15% couples presenting with infertility, male factor infertility accounts for 50% of these couples. Of these 10-20% of infertile men present with azoospermia, that is there is total absence of sperms in the ejaculate.1 These azoospermic men need to be properly examined as far as reversible factors are concerned. Once a detailed history, physical examination and investigations have been completed, some patients might get successfully treated with the use of hormonal therapies, and others will need attempts at surgical sperm retrieval. Testicular sperm extraction by utilizing microdissection(mTESE) is supposedly referred to be a gold standard technique for surgical sperm retrieval, a technique considered that was initially developed by Schlegel PN2 This is a microsurgical method which has acquired the most modern features for retrieving sperms from men with nonobstructive azoospermia(NOA). The clinical factors known to affect reproductive success are testicular histology, karyotype and Ychromosome microdeletions. Testicular histology might be available
occasionally. Four testicular histologies are known to cause NOA. Hypospermatogenesis, which is the least severe form of NOA where one gets a sperm retrieval rate (SRR) of 73-100%, in contrast to late maturation arrest, where SRR is 27-86%,earlymaturation arrest having a SRR of 27-40% and 4th that is sertoli cell only syndrome(SCOS), that is the most severe form of azoospermia, with a SRR of 22.5- 41%.3–6 Following that more number of studies proved mTESE is 1.5 times more efficacious in contrast to the conventional TESE in which multiple biopsies are taken randomly, a technique which has proved to be, twice as effective as testicular aspiration in controlled trials.7 The basis of developing MicroTESE was the finding that spermatogenesis in testis is heterogenous. Most of the individuals presenting with non obstructive azoospermia don’t have germ cells in their seminiferous tubules (STs) and present with a very thin appearance on microscopic appearance which appear to be collapsed. Occasional STs where full spermatogenesis is found to have a greater size and have a deeper colour on microscopic appearance, differentiating them from the collapsed seminiferous tubules and thus chosen from the tubules around them. With this 63% of men had sperms identified by means of mTESE, as compared with the utilization of original testicular sperm extraction(TESE) procedure published to begin with.2 Since then various studies got published on this topic. A systematic review was carried out to specifically check the literature on how to differentiate various forms of azoospermia, laboratory investigations, karyotype findings and various preoperative factors that would help in increasing the success of mTESE, in specific conditions of azoospermia, besides surgical technique and how to process testicular tissue retrieved following mTESE.
We searched Pubmed search engine for articles reported 1997 onwards to 2019, that were published in English and full article was retrieved. We searched research articles regarding azoospermia classification, obstructive azoospermia(OA), Non obstructive azoospermia(NOA) causes including Klinefelters syndrome(KS), those due to Hypothalamo-Pituitary-Axis(HPA) failure like Kallmann Syndrome(KS), other genetic factors, hormonal therapy for treatment, or to improve success of mTESE, Varicocele repair for improvement of sperm status, all human studies, studies were needed to be reporting on how to use methods that ensured full help prior to mTESE in ensuring that sperms were obtained by this surgical procedure. In mTESE studies which were carried out in human patients those studies which were published in relation to Non obstructive azoospermia secondary to failure of the testis outcomes like and sperm obtained, pregnancy rates clinically or total birth rates were documented.
Of the total 716 topics we selected 119 articles relevant to microtesticular sperm extraction, specifically done in human subjects of which, 429 for medical optimization that were in English. As for varicocoele repair and azoospermia 34 were selected being specific for humans and being in English. 102 full texts were selected regarding testis tissue processing, being in English language for final analysis. Of the total ultimately 62 articles got included in the review.
Azoospermia evaluation
A detailed history should be accompanied by a thorough physical examination. With the male history one will be able to outline the various potential and etiological factors causing infertility with associated Azoospermia. Main factors are duration of infertility, coital exposure, besides developmental and childhood history, history of any systemic illnesses, surgical history. A complete physical workup is done with idea behind being to find potential etiology for azoospermia, Examination needs degree of virilization, any obesity, gynecomastia, anosmia for ruling out KS,8 bilateral hemianopsia, for ruling out pituitary tumor,9 any abdominal and inguinal scars. Close examination of phallus, to find any meatal abnormalities along with penile curvature. Testicular examination includes size, consistency and the presence of masses. Palpate both epidydides and vasa deferens for their presence/absence, consistency and nodularity that might suggest obstructive or infectious etiologies. Palpation of spermatic cords with and without valsalva manouevre might show a varicocele and a digital rectal examination help in identifying dilated seminal vesicles or cysts that cause ejaculatory duct obstruction (EDO).10 see Figure 1 for causes of azoospermia. Of the laboratory investigations most important in azoospermic males are semen analysis and certain serum hormones levels that are morning T and FSH being the minimum but also need LH, Prolactin and estrogen. Once semen shows low pH<7.2 or absence of fructose, then EDO or congenital vasal absence needs to be thought off and use transrectal utrsound (TRUS) and physical examination respectively. More investigations are genetic testing like karyotype and the presence of Y chromosomal microdeletions. If FSH IS<7.6mIU/ml and testicular long axis >4.6cm, it predicts OA in 96%of cases, while conversely if FSH is>7.6mIU/ml with a testicular long axis <4.6cm predicts NOA in 89% of cases as per Schoor et al.11 Figure 2 is a decision flow chart regarding how to manage an azoospermic patient. In the case of Y chromosome microdeletions and the finding a particular level of sperm concentration needed to carry out the genetic investigation , a retrospective study was carried out by Johnson M et al.,12 where a total of 1473 subjects ,in whom microdeletions was observed in 58/1473 men representing 4% of the population studied. These microdeletions were separated in the subsequent areas: Azoospermia factors(AZF); 75% showed AZFc, while 13.8% presented with AZFb+C. 6.9% with AZFb, and only 1.7% represented AZFa and partial AZF are respectively. None of them with an AZF had a spermconcentration of >0.5million/ml. A high FSH concentration was found to have very high significance (p<0.001), although low sperm concentration was significant but not that much as high FSH values(p<0.05) to be significant predict that a microdeletion would be found,12 see Figure 1 for causes of NOA
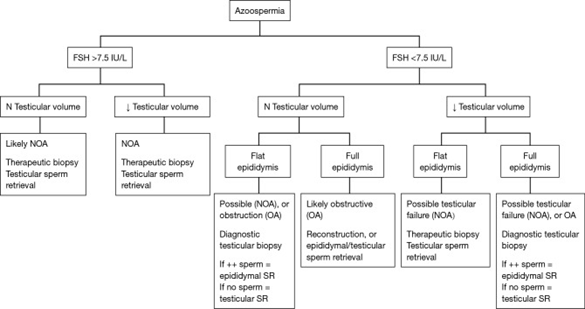
Figure 2 Courtesy.61 Flow chart depicting surgical management of the azoospermic patient based upon FSH and clinical examination. FSH and physical examination are two very important clinical features to determine if the etiology of azoospermia is likely NOA or OA. These findings help the surgeon to determine if a diagnostic or therapeutic testicular biopsy is required; or, if reconstruction or epididymal sperm retrieval is required. When assessing the epididymis, it may feel full & prominent indicating sperm may be present but block by obstruction, or, flat and subtle in which case it is less likely that sperm are present in the epididymal tubules; refer to Table 1 for an inclusive list of the other diagnostic tests important in initially assessing an azoospermic patient such as genetic testing, semen analysis volume & pH etc. NOA, non-obstructive azoospermia; OA, obstructive azoospermia; SR, sperm retrieval.
Technique microdissection testicular sperm extraction
The advances made regarding Microdissection Testicular Sperm Extraction has been done for specializing beyond previously used either single site biopsies or multiple testis biopsies to Microdissection Testicular Sperm Extraction Figure 2. This aids in pointing the seminiferous tubule (ST) to be selected for increasing sperm collection, minimize surrounding testicular tissue loss, thus establishing the mTESE procedure. Originally Schlegel utilized a magnification that was six to 8times for initially identifying and subsequently preventing the compromise of blood vessels below the tunica albuginea once a large transverse incision is made over the tunica albuginea. Once ST’s were seen then for further enhancing their visualization the magnification was increased upto twenty- twenty five so that the ST’s within the most part of testis parenchyma were then seen, with bigger and more deeply coloured ST’s identified and subsequently sharply cut and separated. Thus less tissue was excised in contrast to the earlier large testis biopsy where larger incisions and more tissue were removed (9.4mg vis a vis720mg). Moreover SRR increased by 1.5 fold using mTESE as compared to standard TESE, and leading to 52% overall SR as quoted earlier in the published articles.2 Very few changes were made in the first technique described. A study of 900 men having NOA undergoing 1st attempt at mTESE was reported by Ramasamy et al.,13 in 2013. Of these men 474(52.6%) had successful retrieval of sperms on the 1st mTESE. 65% representing 308/474 of those had sperm found just on the initial large transverse incision alone, while in the rest 35% required to go in depth of the testis for SRR. Further they observed that reported that 8% or 40/506 patients who needed mTESE from both testis due to inability to retrieve sperms from the earlier side were successful in sperm retrieval on the contralateral side.13 If the original incision is not helpful in visualizing the entire testicular tissue of the testis and no sperm were retrieved, in view of dilated tubules(DT), or otherwise tubules with slightly larger caliber (SDT)(X24) than that of the surrounding. Caroppo et al.,14 conducted a retrospective study, in which they suggested to make a second large incision opposite to the original incision. With this technique in 42.8% i.e. 95of 222 patients they got SR. SR was successful in 90% of the testis with DT’s, in 47% with SDT’s and only in 7% of those with non dilated tubules(NDT). They for the 1st time proposed that spermatozoa might be retrieved even for slightly dilated tubules (Figure 3).14
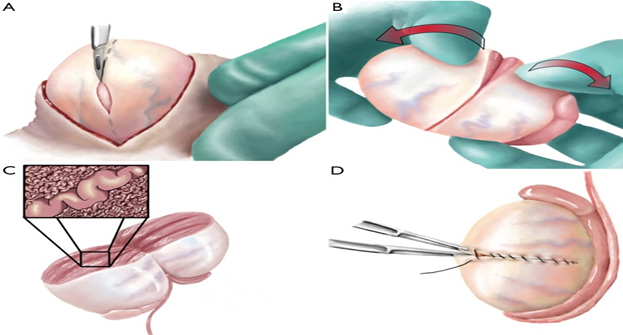
Figure 3 Courtesy.61 During mTESE, the testis is bivalved along the equatorial axis along an avascular plane. Once the initial wide exposure is performed and some sampling of superficial tissue shows no dilated seminiferous tubules, then “dissection lanes” (shown by dark vertical lines in the drawing) are made along avascular testicular lobules, allowing evaluation of every seminiferous tubule through the operating microscope. Only those seminiferous tubules showing dilation are removed for sperm extraction. mTESE, microdissection testicular sperm extraction.
This supported the concept that full spermatogenesis was observed in dilated tubules which may explain why mTESE is based on the normal physiology, further the pattern regarding ST’s removed, along with histology might represent an additional outcome measure of mTESE.14 In another prospective comparator study by Amer et al.,15 where 264 men having NOA were studied supported these findings where sperm retrieval rates of 36.3%(89/245) were found among seminiferous tubules(ST)<300µm in diameter, while 84.2%(16/19) among tubules >=300µm. Thus they suggested best cutoff level of ST diameter is 110micrometer with 86% sensitivity and 74.4% specificity and if ST diameter>300µmeter a single tubule biopsy was sufficient to harvest enough testicular spermatozoa for ICSI/or sperm freezing.15 But recently Yu et al.,16 found findings that were against the above hypothesis with smaller SRR being 3.1% within tubules which were more than 100 micrometre in contrast to 25% in smaller a tubules being under 100 micrometre in patients presenting who with the syndrome known as Sertoli cell-only syndrome(SCOS). They found men having ST diameter >=100µm had a significantly larger testis along with lower FSH than those having<100µm(19.9 vs 8.1and FSH 5.3 VS 25.9m IU/ml respectively. Possibly this was specific for SCOS where aetiology may be different.16 Corona G et al.,17 conducted a new review that included meta-analysis regarding sperm retrieval and results of intra cytoplasmic sperm injection in patients presenting with KS. They included 37 trials from the total of 139 studies which included 1248 patients presenting with an average age of 30 years. 18studies utilized mTESE, while 13 studies used conventional TESE and one case where TESA was used. In 4 studies a mixed approach was used and in one of the study, the approach used for SR was not mentioned. In total a sperm retrieval rate/TESE cycle was 44 (39.8). Same observations were found on trying to compare mTESE with conventional TESE (SRR43 [35; 50%]vis a vis 45[38;50%] vis a vis 45[38;52%] for cTESE and micro TESE respectively. On meta regression it was found that particulars like age, volume of the testis or hormonal parameters that included FSH, LH and Testosterone were not tested at the time when patients got recruited and all these factors influenced the final outcome namely SRR. They also found that vis a vis bilateral procedure differences were not seen in unilateral approach. As far as effect of earlier T therapy was concerned, enough data was not present to find the effect of earlier T therapy on SRR. Following ICSI therapy outcomes regarding ICSI were present in 29 studies. In total out of 410 ICSI cycles, 218 biochemical pregnancies were observed (PR=43[36; 50%]). Just as in SRR, the influence of KS age, mean testis volume, hormonal levels like LH, FSH or total T levels had no influence on either PR or LBR. Enough results were not present for finding out test the effect of women’s age or other fertility conditions regarding pregnancy rate and live birth rates. Finally no difference in PR or LBR was observed with the use of fresh testis sperm in contrast to the utilization of cryopreserved sperm.17
Hashemi et al.,18 tested checked 7 molecular markers in azoospermic men for making an assessment of the degree of SR, of these being 3 premeiotic markers(ESX1,DAZ,DAZL) and 4 post meiotic markers (ZYMND15,PRM1,TNP1 and SPEM. In study of 63 azoospermic men they found expression of post meiotic markers transcripts there were significant decreases in NOA and its subgroups (SCOS,MA,HS) with spermatogenic failure compared to normal spermatogenesis seen in (OA), with an exception of ZYMND15 for the HS group. Thus ZYMND15 marker can be used to differentiate HS from SCOS and MA. There was a significant reduction in post meiotic markers in negative vis a vis positive sperm retrieval group and SPEM1 was the best positive prediction power (85% for micro-TESE outcome out of the 7 markers used.18 Mao et al.,19 further did not find any superiority of doing pre micro-TESE testicular biopsy for HPE regarding results of micro-TESE.19 Thus in summary very little changes have been done in surgical technique since the initial introduction of mTESE. In most of the cases searching exhaustively the superficial seminferous tubules following incising the testis seems to help in achieving sperm retrieval in most of cases; but one needs to carry out an exhaustive search for retrieving sperms in the rest 35% of cases, and in rest 8% of patients where originally sperms were not found doing mTESE in the opposite testis helps in getting sperms in which are negative in original attempt and site. In general Sperms generally get retrieved from the one with bigger tubules chosen on microscopic assessment but Yu et al.,16 report queried this although they were in case of SCOS. SPEM1 is the best predictor of SRR in NOA in mTESEs (Figure 4).
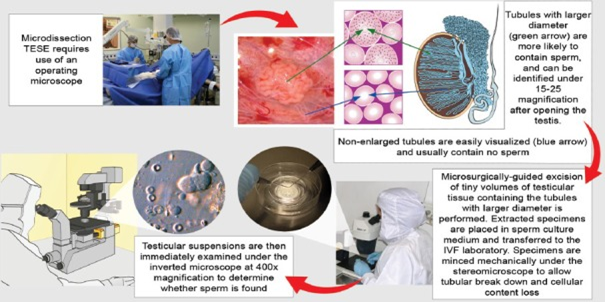
Figure 4 Courtesy.62 Microdissection testicular sperm extraction. The illustration depicts its main technical aspects, including the use of an operating microscope and the identification of enlarged seminiferous tubules, and initial processing of extracted specimens.
Adjuvant hormonal therapy
In 2005, a prospective experience from 3 international centres of 42 patients having NOA (age range25-39yrs) and where histopathologic diagnosis of was made as MA in 42.9% or HS in 57.1% was published by Hussein et al.20 These patients were treated with clomiphene citrate for 3-9mths. They increased the dose from 25mg/d upto 75mg/d for achieving a serum testosterone in serum ranging within 600 to 800ng/dl. 27/42 patients found sperm in the ejaculate once semen analysis was attempted i.e. 63% had positive findings having a mean sperm density of 3.8million/ml (range1-16million sperm/ml). Of the remaining15 patients, SSR was attempted being successful in cent percent i.e. 15/15.20 It seems that this study represents a very particular group of men presenting with non obstructive azoospermia but gave statistically significant increase in testicular biopsy patterns. Reifsnyder et al.,21 found no advantage of using medical therapy prior to mTESE. They conducted a retrospective study of 1054 subjects who had presented with non obstructive azoospermia and underwent mTESE. Out of these 736 subjects had hormonal levels tested preoperatively. Adjuvant therapy was administered for 2-3mths only to those subjects where Serum T levels was below 300 ng/dl prior to mTESE, if T:E2 was <10, an aromatase inhibitor was used (either of the 2 i.e. 50mg or 100mg testolactone b.d. orally or 1mg of anastrazole/d). If T:E2 ratio was over 10, then clomiphene citrate (CC) was preferred and utilized. If the patients did not show response to anastrazole or CC then T, HCG injections got used in dose of 1500- 2000IU administered by s/c route twice or thrice a week in addition. There was not much difference in the results irrespective of treatment regarding SR as sperm retrieval was concerned (51 % vis a vis 61%), pregnancy rates(50% vis a vis 56%) or total birth rates which were born live(38% vis a vis 48%). Moreover sperm retrieval, pregnancy rates or live births were similar irrespective of the response among the men that did or did not respond to treatment in patients having T levels above 250ng/dl. Patients presenting with non obstructive azoospermia and KS in particular, in which medical therapy was used Ramasamy et al.,22 described 68 subjects kept for mTESEs by 91 attempts.22 Men having serum T levels below 300 nanogram/decileter received medical therapy with aromatase inhibitors, CC or HCG. In this group a SRR was 66% (45/68) i.e. in 68%attempts made i.e. 62/91 attempts. They found that no improvements occurred regarding sperm retrieval with adjuvant hormone therapy ,though analyzing further found that men responding to hormonal therapy with a T response of above>250 nanogram/decileter, led to greater SRR in contrast to those having T below 250 nanogram/decileter (77% vs 55%). Fertilization rates were 57% following intracytoplasmic injection giving a total birth of live children in 45% in the subjects where SR was attempted.22
A prospective evaluation was done in 48 men who had previously failed mTESEs by Shiraishi et al.,23 in 2012.23 Further mTESE was to be done for which 28men received medical therapy and 20 weren’t given the medical therapy. Those who got it received daily subcutaneous injections of HCG injections for 4-5mths prior to 2nd mTESE and rFSH 150IU 3 times/week for 3months before repeated mTESE if endogenous gonadotropins decreased during the HCG stimulation. Results showed that the SRR was higher in those that got the medical therapy in contrast to those who did not (21% vis a vis 0%).10 In 2/21patients (10%) sperm was surgically retrieved with a histopathological diagnosis of late maturation arrest (MA) and hypospermatogenesis (HS).23 No comparator control group was there for patients who underwent repeated mTESE alone without hormonal therapy. Shiraishi et al.,24 in the year 2016 further documented a multi institutional Japanese study in which 21 subjects, after ruling those subjects having any chromosomal aberrations, Ychromosome microdelations, very small testis or previous medical therapy 1 month of HCG(5000IU) thrice weekly, patients were given rFSH(150IU) three times a week and HCG for the next 3mths. 13 and 6 patients had SCOS and an early MA after the 1st mTESE. After 2nd mTESE 2 patients (10%) had sperm retrieval. Thus concluding hormonal therapy had limited value 6 though they had not kept any control group.24 A prospective multicenter trial that examined four groups of medical therapy prior to mTESE in men presenting with non obstructive azoospermia was reported by Hussein et al.,25 in 2013. 612men got included in the study, while 116 were used as controls obstructive where received medicines were not administered and the remaining 496 subjects received CC. FSH along with total T were raised in group 1 subjects and remained on CC which was administered to 372 subjects. 62 of the men represented the 2nd group in which testosterone remained low, although they showed an increase in FSH with no or little increase in LH and were supplemented with r HCG.46 of the subjects belonged to the 3rd group where alterations in FSH, LH, T, were not visualized and 16 subjects representing the 4th group showed a continuous reduction in T levels while on CC. Hence CC was interrupted in groups3 as well as 4 with supplementation of HCG and HMG. The amount of medical hormone was kept proportional to those that helped achieving 1.5 times FSH value with the basal, and T levels of maintained around 600-800 nanogram/decileter. In 10.9% patients sperms were found in the ejaculation irrespective of which group the patient belonged. In total the surgical SRR was observed to be 33.6% of mTESE in controls used, that was considerably greater than when the medical therapy was used giving a 57%succesful SRR.25 Absence of blinding and a smaller surgical SRR of in the controls amounting to 33.6% , that was lesser than earlier published studies were the limitations of this study. Control groups of men with who did not receive hormonal therapy was not assessed, thus assessing the influence of medical therapy was not feasible in this study.
Additional hormonal treatment for subjects where previous mTESE failed among 20men, were given 5000IU HCG thrice weekly for a month or 2months prior to attempting another mTESE by Shinjo etal.,26 150IU rFSH thrice weekly was introduced in case FSH dropped below 2mIU/L. Both T levels within testis along with DNA synthesis increased with addition of medical therapy, which was PCNA staining observed within spermatogonia. On attempting mTESE multiple times sperms were obtained in 15% subjects i.e. 3/20 subjects.26 Limitations was smaller study numbers and absence of any controls. 26 men where previous attempt at mTESE had not revealed sperms and those presenting with late MA, was reported by Kobori et al.,27 Twice weekly rFSH was administered for 3 months in a dose of 75 IU and then 150IU twice a week 4-12 moths. Sperms were found in the ejaculate in 19.2 % subjects with this protocol i.e. 5/26 subjects. The results found were a little greater than earlier publications regarding presence of sperms naturally the ejaculate which occurred in 10%- 8%. A lack of control arm, limited their results.
Hu et al.,28 in 2018 using a prospective pilot study gave new directions to therapy. They studied 35 subjects presenting with non obstructive azoospermia where earlier attempts at SR had not produced sperms with the use of TESE, of these10 subjects were kept as a controls while rest 25 men received goserelin around 24 weeks, by which led to a reduction in release of FSH/LH. Subsequently these subjects were given hormonal therapy like 2000 IU HCG weekly for 20weeks along with 150IUHMG twice weekly for 16weeks with the idea of increasing these FSH/LH which had been initially down regulated using Gn RH agonist. But it seems this hypothesis did not appear to work as just two of the 25 subjects presenting with HS had sperms found in ejaculate.28 Limitations being that Hu et al.,28 did not use any subjects as control not receiving these hormonal therapies and their findings regarding SR were much smaller than found in earlier studies. Thus summarizing, there is limited evidence that supports the use of additional medical therapy. Moreover what therapy should be used in men having non obstructive azoospermia along with in how much dose and for how long still is not clear unknown all these studies, inspite of their being a physiological basis regarding use of these hormonal therapies? Still Hussein et al.,25 studies, despite having its limitations does favor the use of additional hormonal treatment in subjects having non obstructive azoospermia before the microdissection sperm retrieval.
Varicocele repair before mTESE
Operating on Varicocele prior to mTESE subjects having non obstructive azoospermia has been preferred by some if there is a clinically evident varicocele. In a meta-analysis performed by Esteves et al.,28 in 2016 on examining the importance of varicocele in the etiology of subjects having non obstructive azoospermia they carried out 18 studies that had 468 subjects having non obstructive azoospermia along with a varicocele. Of these 7 prospective and 11 retrospective studies got included .The procedure used in 13 of the studies was inguinal and sub inguinal microscopic varicocele repair (VR),29–41 one used macroscopic inguinal VR42 and in 3 studies percutaneous embolization was employed for treating varicocele.43–45 Out of these 3 studies had kept a control group regarding comparison of ultimate results. In the study carried out by Inci et al.,41 30 subjects having non obstructive azoospermia, no VR was done for reasons not cited prior to mTESE and they did not cite why mTESE was attempted while 66 patients already had a previously successful varicocelectomy and despite similar basal factors, that were tested in both groups, selection bias was still apparent and the treated group had a much higher SRR(53%vs 30%) with not much changes in 2ProNuclei fertilization rate(63.9% vs 53.6%).41 The time interval between VR and mTESE was around 23.6mths. In Haydardedeoglu et al.,42 study which was a retrospective study done in 2010 the control group(n=65) were subjects who had mTESE without any VR on while the intervention group had undergone a macroscopic VR(n=31) for varicocele classified as grade 3 at some time before mTESE. The mean interval of mTESE was 42mths in the group achieving pregnancies had mTESE around 42 months before the pregnancy in comparison to those not getting pregnant this gap was versus 80months. They used minimum Inclusion and exclusion criteria in this study.42 A prospective trial was reported by Zampieri et al.,39 in the 3rd observational study, where 35men having non obstructive azoospermia NOA and a grade 3 varicocele repair group 1(n=19) had VR 3mths before mTESE versus group 2(n=16), where VR and mTESE were done concurrently. Whether the study was randomized was not clear. From the methods and detailed as the methods and discussion this was not clarified. If it was an study where which treatment was to be done was not provided and thus a great risk significant risk of bias based on patient selection and treatment was present. However the SRR was much higher in the first group 1(57.8%) in contrast to the 2nd group (27%).39 Ultimately in all the 3 studies SRR was much higher with odds ratio(OR) 2.65,95%CI 1.69-4.14, as did Pregnancy rates in favor of performing VR although results did not reach statistical significance with OR 2.07,95%CI0.92-4.65 and Live Birth rates as well a favored of VR with OR 2.19,95%CI0.99-4.83;P=0.05.15 additional studies described VR in subjects with non obstructive azoospermia with in which no controls were kept found sperm in the ejaculate post surgically among 43.9% ranging from 20.8-55.0%. In 13.6 % pregnancy resulted just following use of assisted reproductive technology(ART) i.e. IVF/Intra Cytoplasmic Sperm Injection.45 Maximum sperms on the basis of NOA histopathology, sperms returned to the ejaculate in men with HS(56.2%) which proved to be statistically significant higher than in MA (35,3%;OR2.35,95%C1,04-5.29) and sertoli cell
–only syndrome(9.7%;OR12.0,95%CI4.34-33.17); Sperm in the postoperative ejaculate was also more for maturation arrest compared with sertoli cell–only syndrome (OR5.09,95%CI1.83-14.10).32 Higher the varicocele grade higher the rates of sperm in the ejaculate were found (grade 1 :7.7%;grade II:25.8%,grade 3:34.3%) although results did not correlate with statistical significance.
In this meta-analysis, selection bias in identifying studies might have been there.46 e.g. the study by Schlegel & Kaufman47 was not taken into account. A big retrospective clinical study that was retrospective in nature was undertaken and presence of sperm in the ejaculation was documented, and 7/31(22%) had sperm reported on at least one semen analysis postoperatively, while varicocele remain could prevent TESE in only 3/31(9.6%) of varicocele repaired men, and SRR for men with clinical varicocele was same, independently from whether earlier varicocele repair had been performed.47 Another meta-analysis was done by Kirby et al.,48 where they examined the role of VR in men presenting with oligozoo or azoospermia prior to ART.2 studies where men had azoospermia was included. They found VR resulted in higher pregnancy rates (OR2.336) and higher sperm retrieval rate(OR2.509)in the persistently azoospermic group. Thus both oligozoospermic and azoospermic patients VR gives a higher live birth rates and PR’s with IVF and ICSI.48 Of the two studies that Esteves et al.,42 had included the one carried out by, Haydardedeoglu et al.,42 was a retrospective one involving of subjects with non obstructive azoospermia kept for to undergo an intracytoplasmic sperm injection cycle, 31 subjects with non obstructive azoospermia had a plan to undergo VR were compared with 65 subjects with non obstructive azoospermia scheduled to undergo an intractoplamic sperm injection. Among the VR group the sperm retrieval rate was significantly more (60.8%vs 38.4%), as was the clinical pregnancy rate (74.2%vs52.3%) and live birth rate (64.5% vs 41.5%).42 In the 2nd paper included in this meta-analysis was conducted by Inci et al.,41 in 2009, which was a retrospective report of 96 subjects with non obstructive azoospermia and without accompanying varicocele. 66 out of these received a surgical varicocele correction while 30 did not. Greater SRR was found among men who had surgical varicocele correction (53% vs22.2%).41 Two-pronucei FR was similar(63.9% vs 53.6%)as was the CPR(31.4% vs22.2%).27 Since these 2 meta-analysis no more studies testing surgical varicocele correction in subjects having non obstructive azoospermia were reported. For differentiating the transcriptomes of testis which was responsive to varicocelectomy and those that were not for finding the predictors of sperm in the ejaculate Shiraishi et al.,49 carried out a study in 2017. 83 with subjects having non obstructive azoospermia accompanied with left varicocele on the left study had a varicocelectomy by microsurgical approach with biopsy of the testis in the same sitting. Following varicocelectomy, sperms were obtained in 20 patients(24%) within 12mths, that included 2% patients having SCOS, i.e. 1/43, (37%)with MA i.e. 10/27 and 69%)with HS i.e. 9/13subjects. Group comparison of 23,003genes within men having sperms or those not having them within the ejaculate of men with MA showed up regulation of several genes which were related to cell cycle while down regulation of those genes that were responsible for antioxidant effects in subjects where sperms could be retrieved. Expression of Proliferative cell nuclear antigen (PCNA) was markedly high in the10 subjects who responded to varicocelectomy in contrast to the 17 nonresponsive subjects. Thus get a better insight in how varicocele affects spermatogenesis.49
Summarizing, sperms found appearance in the ejaculate is associated with VR and might give a higher surgical SRR. But various study biases are present that include minimal studies used as controls further bias in recruiting patients for undergoing varicocele repair. Besides that in view of all of the men being azoospermic prior to VR, thus the only post-operative finding that can be observed is recovering sperms from ejaculate. Moreover earlier studies that 10- 18% of subjects with non obstructive azoospermia have sperm found in ejaculate are there for a very little time despite no surgery done for varicocele.
Further preparation of tissue obtained from testis
The Tissue that gets removed at mTESE is transferred to human tubal fluid medium (for example) where addition of 6% plasmanate has been done.1 Subsequently both mechanical and enzymatic methods are used to maximize sperms obtained. These different techniques include tissue shredding, squeezing the tissue, utilize a cell strainer or tissue grinder, RBC–lysing buffer, pentoxifylline along with collagenase. After obtaining testis biopsies from 15 subjects having non obstructive azoospermia and 5 from OA, Nagy et al.,50 transferred tissue obtained in Earle Medium containing 3ml HEPES buffer in which 2.25% human serum albumin had been added in a solution form. Following that this testicular tissue was dried as well as squeezed in the Earle medium solution and subsequently underwent centrifugation. The pellet thus obtained following centrifugation had addition 2-4nml RBC lysing buffer (155nmol/LKHCO3, and2mmol/L ethylenediamine tetracetic acid:pH7.2) and kept for 10 minutes before further addition of aforementioned sperm medium. Repeated washings and centrifugations were done till the final pellet that was resuspended, containing both sperms and testis cells were obtained. Finally in this study 46.7% sperms were obtained in subjects presenting with non obstructive azoospermia i.e. in7/15 subjects. They then divided the testis samples among 5 subjects having OA, and used sperms obtained via standard protocol for intracytoplasmic sperm injection in half of the oocytes the other half with sperms obtained by means of RBC lysis approach. Results were same whichever method was used in terms of pronuclei formation; embryo formation and how many embryos got transferred. But limited numbers and since the study was done without controls for comparison for SRR in non obstructive azoospermia biopsies from the test is limited this study.50 To increase the number of sperms from testicular biopsies in subjects presenting with non obstructive azoospermia Ostad et al.,51 in 1998 presented additional methods. Of the 81 subjects presenting with non obstructive azoospermia that were to undergo TESE they tried comparing the sperm yield with standard tissue mincing in medium with iris scissors vis a vis further processing by tissue passage through a 24 gauge angiocatheter in 20 of these patients. A 470% increase resulted with the use of angiocatheter with a yield of 390,000 sperms in contrast to 83,000 with standard protocol.51 In another study carried out Ozkavukcu et al.,52 in 2014 a series 20 subjects presenting with non obstructive azoospermia in whom mTESE were planned testis tissue was inserted in medium used for sperm washing, and were broken with the use of two 26 gauge needles with the idea of two stretching and tearing the ST’s until no thread like tubules existed. This tissue was then divided into 2. For the 1st one the minced tissue and solution was placed into a 2 layer density gradient medium(Puresperm40/80), and for the 2nd group only the fluid obtained following mincing was sucked and transferred into the centrifuge tubes for centrifugation. After centrifuging at 10minutes at 400g the pellet was washed with sperm separation medium by which RBC’s could get separated, and then washed using medium meant for sperm washing. The total number of sperms obtained had better quality and quantitatively were more a(60.2 vs 39.4 cells) in the group where only the fluid was selected for centrifugation as compared with both tissue as well as fluid. Moreover in 2 subsequent patients sperms were found from the fluid that had been aspirated than with the injection of the tubular fragments, which suggested that the remaining fragments of the ST’s might block the identification of sperms.52
A newer method utilized by many reproductive centres is the use of Enzymes for separating testicular tissue. Extracellular matrix gets disrupted and digested by enzymes and thus the sperms along with cells get removed from the ST’s. This has been known for the last 40 years; with the 1st pregnancy following enzymatic treatment being obtained in 1996.53 Crabbe examined utilization of enzymes in 4 types of ways in 1997. Collagenase type 1A, collagenase type 4 or, collagenase type 1A in addition to elastase or collagenase type 4 with elastase were tried. On comparing the SRR on 10 testis biopsies obtained from patients where orchiectomy had already been done they found that instead of type1collagen. Type 4 collagen alone or in combination with elastase yielded highest amount of SR from 100mg of tissue taken from the removed testis that was incubated with collagenase over an hour. Similar vitality among spermatozoa was seen between collagenase treatments and control samples, and motility was similar between type 4 collagenase and control samples, while Type 1A collagenase impaired motility significantly as compared with control and type 4 collagenase. They further evaluated type 4 collagenase in testis biopsies in men with NOA. Crabbe et al in 1998 presented a total of 41 patients with TBs secondary to non obstructive azoospermia, where no sperms were found following mechanical mincing of the tissues. Further they treated the TB biopsies with RBC breaking buffer, and sperms were found in 14 TB. In the remaining 27/41cases type 4 collagenase was used to separate the tissue from the sperm and successfully sperms were identified in additional 7/27 biosies. A prospective study of 177 subjects presenting with non obstructive azoospermia underwent mTESE, earlier TB were treated with mechanical breaking utilizing mincing and RBC breaking medium. In 36% i.e. 65/177 sperms were retrieved. In the remaining the 112 TB that had failed were incubated for an hour with collagenase type 4 along 25µl DNAse, and further 33% i.e.37/112 accounted for sperms .Approximately in 30’ following treatment with enzymes s perms were obtained. A total of 52% SRR and 36% clinical PR was found in these patients with intracytoplasmic injection.54 Further Modaressi tested TB obtained from 150 from subjects having non obstructive azoospermia where no sperms were found using of mechanical breaking despite 30’ of looking under the microscope. Rest of the TB was treated with use of combined collagenase type 4 along with DNAse and, that gave another 9% of i.e. in 13/150.55 Important was the observation of Crabbe et al. noting that testicular sperms obtained remained alive outside even for 3-5 days after removal thus one can plan doing mTESE, just a day or even earlier before planning oocyte pick up. Further Baukloh V56 in 2002 reported on behalf of the German society of reproductive endocrinology and biology, a retrospective where they compared how much success was obtained on intracytoplasmic injection whether enzymatic breaking was used or mechanical breaking. They obtained reports from 11 setups that had performed a TESE on 549 subjects by which, 839 intracytoplasmic injection procedures were performed and they found neither any effect on clinical PR or LBR. Although FR’s were similar in both groups ,on further trying to assess they found higher FR from sperms that were motile obtained by mechanical method of breaking in contrast to use of enzyme digestion ,further even from immotile cryopreserved sperms along with spermatids that were elongated he reported a higher FR’s. Utilization of Enzymatic digested TB’s yielded higher number of embryos which were also of better quality using fresh sperms in contrast to those obtained from cryopreserved samples use of mechanical method was superior.56 It might be possible that ultimate results were influenced by some of basal properties and against clinical setting. Pentoxyfylline has been used to increase motility of sperms that have been retrieved by TB. This allows selection of motile sperms required for intracytoplasmic injection. Pentoxyfylline inhibits breakdown of cAMP in view of it having the property of inhibiting the enzyme phosphodiesterase, that promotes mobility of sperms.57 In 77 subjects where motile sperms could not be found on TSA or TESE, Pentoxyfylline was transferred the buffer for 20’s at a temperature matching the room temperature with the idea of incubation. Then 2 groups were retrospectively formed, one not getting pentoxifylline, and used immotile sperms for oocyte injection via ICSI, in contrast to those who got Pentoxyfylline therapy along with motile sperms utilized for ICSI. A higher FR was observed in the Pentoxyfylline group(66.7% vis a vis 46.4%), greater embryos formed in each cycle(4.7 vis a vis 2.7) and similar clinical PR’s in a cycle (38.3%vis a vis 26.7%) just as the rates of delivery rates and ongoing pregnancies(31.9% vis a vis 26.7%). Further time used for tracing the motile sperm was reduced 4-fold (30min Vis a Vis 120 min).58 The study being a retrospective one along with unaccounted variables responsible for differences between therapies. Some different methods introduced included a squeezing method where squeezing was utilized by few workers .The ST’s are removed following which after transferring in a culture medium, they were and chopped in 1-2 cm lengths. With the use of Pasteur pipettes pressure is put from one end of the ST to another, with the idea of squeezing the tubules to get the intraluminal contents pushed within the medium .Subsequently The medium was then sucked for sperm.59 Different workers tried Glass pipettes for grinding the ST’s, and then centrifuging in cell solution and the pellet getting further suspended in medium ,and then trying to locate the sperms this cell containing solution is then placed through a cell strainer.59,60 Thus summarizing, not good evidence was obtained from most studies using variety of tissue preparation techniques and very few studies conducting comparisons between mTESE tissue processing techniques. Yet it was found that mechanical mincing and subsequent type 4 collagenase and DNase therapy consistently helps in getting yield of sperms from more TB’s in a shorter spent time. Pentoxifylline can be utilized with purpose of increasing sperm motility, which will aid the embryologist to select viable and motile sperms. In non obstructive azoospermia subjects sperms are present within ST’s in the testis. To obtain those sperms, mechanical disruption of the ST’ is needed, which followed by enzymatic digestion might further help in retrieving along with identifying sperm for preparation of intracytoplasmic injection.
None.
The author declared that there are no conflicts of interest.
None.

©2020 Kaur, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.