MOJ
eISSN: 2379-6162


Research Article Volume 13 Issue 1
1General Surgeon, University of Manitoba, Canada
2 Professor Emeritus of surgery, College of Medicine, University of Saskatchewan, Canada
3Professor Emeritus of Anatomy and Cell Biology, College of Medicine, University of Saskatchewan, Canada
Correspondence: Dr. Adebola Obayan, Professor Emeritus of surgery, University of Saskatchewan, Saskatchewan Health Authority, Canada, Tel (306) 261-5659
Received: January 24, 2025 | Published: February 7, 2025
Citation: Obayan A, Keith R, Griebel R, et al. Impact of trauma severity on oxidant/antioxidant status in injured patients during the first seven days. MOJ Surg. 2025;13(1):12-16. DOI: 10.15406/mojs.2025.13.00285
Trauma is an important cause of death due to a combination of over-stimulation of the innate immune responses and a subsequent immune-suppression. Neutrophils are recognized significant contributors to the innate immune response following injury. However, the impact of the severity of trauma, or the type of trauma on time dependent assessment of neutrophil activity and subsequent plasma antioxidant system is not known.
Aim and objectives: Therefore, this study was designed to understand the effects of trauma severity on time dependent neutrophil count and activity following Also, we sought to identify the peak neutrophil activity following traumas and to relate neutrophil activity with changes in plasma free radical production in the same subset of trauma patients and to relate these changes with the antioxidant concentrations observed post trauma.
Methods: A prospective study involving 120 trauma patients was carried out and these study participants were classified into different trauma severity groups based on international classification system of trauma into severe, moderate and mild categories as well as the type of trauma causing the The blood neutrophil count, activity, red cell glutathione concentration and the peroxide concentration in the plasma were assessed over 168-hour period.
Results: Severe trauma was associated with significant increase in neutrophil count, and red cell glutathione depletion. Head injured patients also showed significant depletion of the red cell glutathione content more than other trauma Furthermore, plasma peroxide level peaked 12-18 hours post traumatic event.
Conclusion: We conclude that severe trauma and head injury have a significant impact on plasma oxidant and antioxidant status which may have prognostic implications in trauma
Keywords: antioxidant, head injury, severe trauma, free radicals, glutathione, neutrophil activity
Trauma is one of the leading causes of death in humans, due to a combination of over-stimulation of the innate immune responses and a subsequent immune-suppression which promotes an increased susceptibility to infection and multi-organ dysfunction syndrome (MODS).1,2 Predicting the degree of these immune mediated injury following trauma remains a difficult task.3 Polymorphonuclear neutrophils (PMNs) make up the largest fraction of white blood cells that are recognized significant contributors to the innate immune response following injury.1 In fact, PMN are recognized as the major immune cells that contribute the first line of response following trauma.4 This response often is seen in form of an increased neutrophil count, which occurs primarily from increased margination of neutrophils following trauma rather than an increase in the bone marrow neutrophil production.3 Furthermore, trauma induces neutrophil activation, the facilitation of emergency granulopoiesis and recruitment of naïve cells, which significantly contributes to neutrophil heterogeneity observed in trauma.5 Trauma initiates the migration of neutrophils into injured tissues through the release of cytokines and chemokines.1 These molecules also provoke the activation of neutrophils to cause secondary tissue damage through a host of toxic enzymes, generation and release reactive oxygen species.1 Therefore, PMN play a primary initial role in tissue injury and in the etiology of oxidative stress arising from trauma.6–8
Moreover, PMN count and activity are both important in trauma response, and they have been shown to have a direct relationship to the degree of immune responses triggered by trauma.1 In fact, a direct correlation between neutrophil count and the severity of injury was reported by Akkose et al.9 The greater the severity of the trauma, the higher the neutrophil count. Hence, Neutrophil count is suggested as a prognostic indicator after trauma. Previously, Rovlias et al concluded from their study that white blood cell counts at the point of admission could serve as a strong indicator of injury severity among head injured patients.10
Neutrophil activity is another important indicator of trauma response. Activated neutrophils generate and release ROS through an overactive NADPH oxidase enzyme as well as promote the activity of a tissue toxic enzyme, Myeloperoxidase. Myeloperoxidase (MPO) or circulating plasma levels of MPO has been suggested as a more specific indicator of neutrophil activity.11,12 MPO is a major protein found in the azurophilic granules within the neutrophils, constituting about 5% of the dry weight of the neutrophils.
The impact of the severity of trauma, or the type of trauma on time dependent assessment of neutrophil activity and plasma antioxidant system is not known. Does trauma severity affect neutrophil activity and if yes how? These are some of the unanswered questions that we sought to investigate in this study. Therefore, this study was designed to understand the effects of trauma severity on time dependent neutrophil count and activity following trauma; to identify peak neutrophil activity following trauma; to relate neutrophil activity with changes in plasma free radical production in the same subset of trauma patients and to relate these changes with the antioxidant concentrations observed post trauma.
A prospective study was designed to recruit eligible patients from the Royal University Hospital, Saskatoon over a period of three months (March-May 2003). All trauma patients (n=120) who were over 15 years of age who were assessed for trauma severity and admitted to the health facility were included in the study. An informed consent was obtained from each study participant before they were enrolled in the study. Trauma assessment was done by the trauma team using the Injury Severity Score (ISS).
Sample collection and analysisBlood samples were obtained upon admission via a venipuncture of the median cubital vein, whenever possible. Blood samples were fed into the automated blood sampler to obtain the neutrophil count of each study participant. Plasma was promptly separated from the blood samples by spinning the whole blood sample collected at 3000 rpm for 10 mins and thereafter, carefully removing the top supernatant into an Eppendorf tube. Plasma samples obtained were promptly stored at -80oC for further analysis.
ReagentsAll reagents used were of analytical grade and purchased from (Sigma Aldrich, Canada).
Determination of myeloperoxidase concentrationNeutrophil activity was measured with the Myeloperoxidase and EnzChek elastase assay kit (Powers, Gupton et al. 1977; Stein and Trainor 1986). Briefly plasma was mixed with Solution A [0.5% hexadecyltrimethylammonium bromide (HTAB) in 50mM Potassium phosphate buffer (pH6.0)]. The mixture was centrifuged at 40C for 15 minutes. The supernatant was decanted and 6.7ml of the supernatant was mixed with 200µl of Solution B [50ml of 50mM Potassium phosphate buffer (pH 6.0) mixed with 8.35mg of o-dianisidine dihydrochloride and 25µl of hydrogen peroxide] in a microtiter plate on a shaker. The samples were read at 460nM in a spectrophotometer as a kinetic assay every 11 second for 5 minutes.
Determination of neutrophil count and neutrophil activityNeutrophil count was carried out by the royal University Hospital laboratory and the data was obtained from each patient's laboratory report.
Determination of erythrocyte glutathione (GSH) concentrationErythrocyte GSH concentration was measured using Asensi's13 modification of the method by Brigelius et al.14 Briefly, whole blood was centrifuged at 300 rpm for 5 minutes at 4oC and plasma promptly separated. Ice cold 30% trichloroacetic acid (TCA) (0.5 mls) was added to 0.5 ml of red blood cells obtained from the spin cycle and gently vortexed. The mixture obtained was centrifuged at 15,000 g for 5 min at 40C. GSH concentrations was determined by reading the absorbance of the supernatant at a wavelength of 340 nm.
Determination of plasma and urine oxidant status using the oxistress assayPlasma and urine oxidants measured with the Oxistress assay.15 Briefly, a 1 in 10 or a 2 in 10 dilution of plasma or urine, respectively, were made up in a tube containing 900 µl or 800 µl of Oxistress reagent consisting of {Thiobarturic acid (TBA) (50 mM), ferrous sulfate (1 mM) and deoxyglucose reagent (100 mM)}, respectively and gently vortexed. Hydrogen peroxide with concentrations of 10, 25, 50, 80,100 mol/l were used as standard. Color reaction in the tube was read at a wavelength of 532 nm after 5 minutes of standing using a spectrophotometer. Plasma or urine oxidant concentration was calculated by extrapolating the absorbance values of unknown samples on a standard curve generated from the concentrations of the hydrogen peroxide standards.
Statistical analysisData were presented as mean ± SEM and presented in tables and graphs. Statistical comparison between groups was carried out using two – tailed students’ t-test. A p value of less than or equal to 5% was set as the acceptable level of significance.
Time dependent changes in the concentration of red cell glutathione levels for each trauma category over a 7-day period is summarized in Figure 1. The red cell GSH concentration decreased over the first 24-hour period following the traumatic event in the severely injured patient unlike the mild and moderately injured patients. The decline in GSH concentration in the red cells reached a nadir between 18-24 h after the traumatic event and gradually recovered dramatically over the next 6 days (Figure 1A & 1B). This decline suggests an increase consumption of GSH by reactive oxygen species (ROS) possibly arising from increased neutrophil activity. The mild and moderately injured patients did not show a similar depletion of their red cell GSH content over the period of 7 days that they were studied (Figure 1A & 1B).
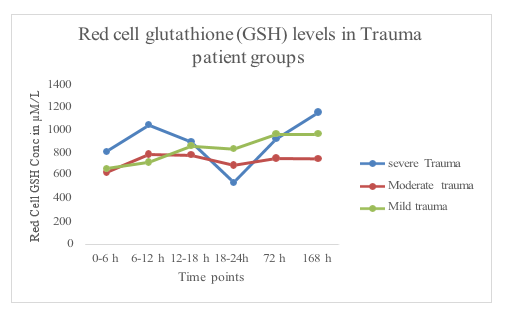
Figure 1A Red cell glutathione levels at different time points in relation to different levels of trauma severity.
Figure 1B Red cell glutathione levels at different time points in relation to different levels of trauma severity.
The effect of the type of injury on red cell glutathione concentration is summarized in Figure 2A & 2B. Head Injured patients exhibited a significant decline (p = 0.041) in the red cell glutathione levels which peaked at 18-24 hours post trauma followed by a sustained significant (p =0.001) rise from 72 hrs till day 7 (Figure 2A & 2B). Moreover, patients with body injury did not experience a significant (p = 0.127) rise in red cell GSH concentration even though there was an initial increase that later declined over the next 6 days and achieved higher than initial red cell glutathione levels by the seventh day. On the other hand, isolated spinal injured patients demonstrated and maintained a continual rise in red cell glutathione levels throughout the study period.
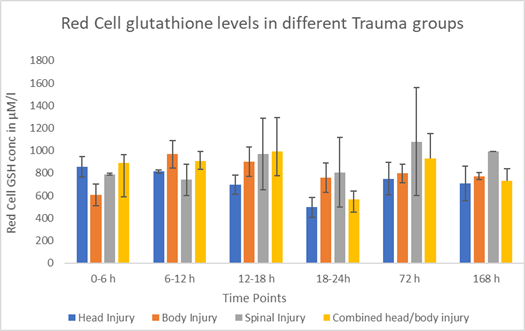
Figure 2 Red cell glutathione levels at different time points in relation to different types of trauma.
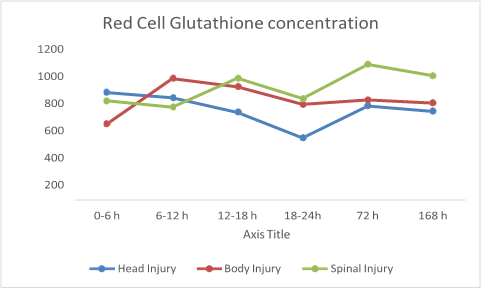
Figure 2B Red cell glutathione levels at different time points in relation to different types of trauma.
The neutrophil count in the mild moderate and severe trauma were generally significantly higher than in normal subjects in the first six to twelve hours of the traumatic event (Figure 3). Furthermore, neutrophil count in the severely injured was significantly (p < 0.05) higher than the neutrophil count in mildly or moderately injured subjects or patient groups in the first 6 – 12 hours of trauma see (Figure 3A). The study of the effect of type of injury on neutrophil count in the first 6-12 hr did not show significantly (p >0.05) higher counts in head injured patients compared to the isolated body injury patients or spinal trauma patients (Figure 3B).
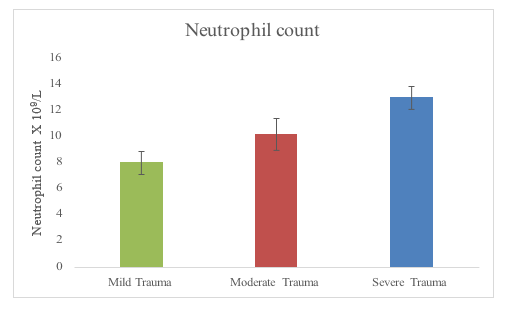
Figure 3 Neutrophil count at 6-12 hours after the traumatic event in relation to different levels of trauma severity.
Plasma MPO activity was observed to have increased following trauma in injured patients (Figure 4). There were two peaks in myeloperoxidase activity between the 6 and 24 hours after the occurrence of the traumatic event compared to controls. A higher peak was observed within the first 7 to 10 hours post trauma while a smaller peak was observed within 13 to 16 hours post trauma. MPO concentration returned to basal levels after 18 hours of injury (Figure 4).
Effects of time and trauma location on neutrophil activityThe effects of duration and trauma location on neutrophil activity is shown in Figure 5 & 6. Neutrophil activity increased amongst the head injured versus the spinal injured (Figure 4). Additionally, neutrophil activity continued to increase over the duration of the study (Figure 5).
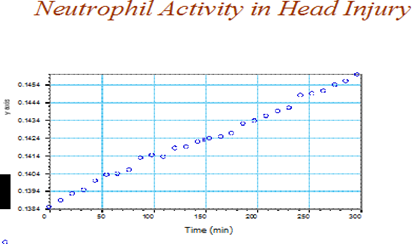
Figure 5 Relationship between post trauma duration and neutrophil activity amongst the head injured.
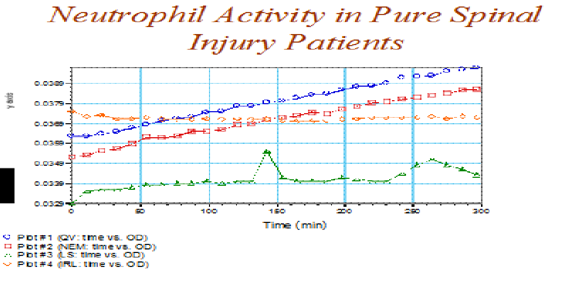
Figure 6 Relationship between post trauma duration and neutrophil activity amongst the participants with spinal injury.
To evaluate the plasma oxidant status, the plasma peroxide concentration was measured, and it exhibited the highest peak between 12 to 18 hours post trauma and was observed to decrease subsequently (Figure 7). Plasma oxidant levels were lowest at 6 hours post trauma.
Trauma has been regarded as one of the leading causes of death in humans due to a combination of over-stimulation of the innate immune responses and a subsequent immune-suppression resulting in an increased susceptibility to infection and multi - organ dysfunction syndrome (MODS).1,2 Predicting the severity of the trauma helps with management, prognosis and patient disposition. This prospective study investigated the effects of trauma location and severity and duration post-trauma on parameters ranging from neutrophil (a major player in immune response) count and activity to oxidant status and trauma-induced free radical production in the injured.
Participants with either head or spinal injury with no signs of infection, following obtaining informed consent were classified using the ISS into mild (ISS < 8), moderate (ISS: 8 - 15) or severely injured (ISS > 16) (Baker et al., 1974, Copes et al., 1988).16,17 We observed an increase in neutrophil count and activity with time post injury irrespective of the location of the injury (Figures 3–6). Polymorphonuclear neutrophil count and activity have been shown to have a direct relationship to the immune reaction triggered by trauma.1 Furthermore, an increased leucocyte count which occurs primarily due to an increased margination of neutrophils following trauma and not necessarily due to an increase in the bone marrow production has been described following trauma.3 In the present study, two neutrophil activity peaks, measured as MPO concentration in plasma irrespective of the injury score, were observed, the first between 7 hr and 10 hr and a second smaller peak between 13hr and 16 hr post injury (Figure 4), thus lending credence to the observations from the previous studies.
MPO is a protein product of chromosome 17 in humans and is found within the neutrophils’ azurophilic granules.18 MPO has been shown to be released following any event that stimulates neutrophil activation and granule-phagosome fusion such as trauma.19 A two-peak increase in the MPO concentration observed in the present study occurred as a result of the exposure of the participants to known trigger neutrophil activation, in trauma. Following trauma, increase in neutrophil count coupled with neutrophil degranulation leads to an increase in MPO concentration and activity which leads to rapid generation of reactive oxygen species.
Increased production of reactive oxygen species has been observed following various aetiology of cellular and tissue damage including physical (trauma), chemical, and inflammatory causes.20 Increased ROS generation lead to the consumption of antioxidant defense systems proteins. Superoxide dismutase and glutathione are two important antioxidant enzymes found in the body that mop up the oxidants and free radicals found in circulation.21 The increased ROS production ultimately leads to oxidative stress, a process that causes oxidation of proteins, carbohydrates and nucleic acids. Oxidative stress arises as a result of loss of the balance between oxidation and antioxidant systems.22,23 This balance is disturbed by trauma because activated polymorphonuclear neutrophils generate and release substantial amounts of reactive oxygen species after trauma.24 In the present study, there was an evidence of increased pro-oxidant activity following trauma as shown by an increase in plasma oxidant peroxide concentrations which was highest in the first 24 hours following trauma (Figure 7). This increased pro-oxidant activity occurs within the same 24 hours in consonance with a depletion of the red cell glutathione concentration within the same 24 hours. This suggests that oxidative stress was highest during the first 24 hours in these injured patients particularly in severely injured trauma patients.
Although several schools of thoughts exist about the relationship and usefulness of neutrophil activity in prognosticating trauma, we have shown here that there is an increase in neutrophil count and activity, increased MPO, peroxide and erythrocyte glutathione concentrations following severe trauma, as well as spinal and head injury. Although these are preliminary studies, it is apparent that trauma severely impacts the oxidation/antioxidation systems in patients, but we don’t know how these affect the outcome in these patients.
The current study was carried out as a pilot study to investigate how time after a traumatic event, trauma severity and the type of trauma affects, neutrophil count and activity, increased MPO, peroxide and erythrocyte glutathione concentrations. Our future directions will be to explore how these parameters and other important components of the oxidant/antioxidant system affects health outcomes in trauma patients and how these can be used in predicting outcome or in the early initiation of certain treatments that may aid recovery and improve outcome. We would also be comparing the results obtained between trauma patients with age and sex-matched controls as this will strengthen our findings and help us draw better conclusions. Furthermore, our future studies will focus on using a larger sample size to investigate a more detailed relationships that exist between neutrophil activity/count, patients’ disposition and physical characteristics.
This is the first study that looks at the role of trauma on oxidative stress. It reviews the effect of the severity of the trauma and the affected organs on oxidative stress. The study shows that initial increaser in the white cell count and the release of it reactive oxygen species measured as myeloperoxidases varies based on trauma severity which is in severe trauma as measured by the trauma scores. The study also shows that we could measure the impact of oxidative stress in humans by comparing the measurable balance of the effect of the free radical release on glutathione and protein carbonyl and the attempts by the body to prevent damage as measured by antioxidant level. Finally the study introduces novel bedside measurement of oxidative such as urine carbonyl and the oxistress assay which can make oxidative measurement available in clinical setting.
†Dr Roger Keith: He was my PhD clinical supervisor and reviewed the data and final document writing the manuscript or providing critical revisions that are important for the intellectual content; Dr Bernard Juurlink: Writing the manuscript or providing critical revisions that are important for the intellectual content; Review the data and final write up. Dr Robert Griebel: Approving the final version of the manuscript.
None.
The author declares that there are no conflicts of interest.

©2025 Obayan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.