MOJ
eISSN: 2574-9773


Short Communication Volume 2 Issue 1
Instituto de Macromoleculas, Universidade Federal do Rio de Janeiro, Brazil
Correspondence: Fernando G de Souza, Universidade Federal do Rio de Janeiro, Cidade Universitaria, Av. Horacio Macedo, 2.030, Centro de Tecnologia-Predio do Bloco J CEP 21941-598-Rio de Janeiro-RJ-Brasil, Tel 005521-3938-7766
Received: October 28, 2017 | Published: February 19, 2018
Citation: Moraes RS, Ricardo N, Saez V, et al. Synthesis of magnetic composite of poly (butylene succinate) and magnetite for the controlled release of meloxicam. MOJ Poly Sci. 2018;2(1):39-42. DOI: 10.15406/mojps.2018.02.00044
Most recent pharmaceutical studies are focused on controlled drug release systems with the advantages that they can offer.1 Among them, on-off delivery systems are especially advantageous, because the drug can be released just in the desired moment since the response is initiated by an external stimulus like a magnetic or electric field, ultrasound and near-infrared light. On the other hand, the combination of therapies is beneficial to treat some diseases like cancer. An exciting alternative is to combine magnetic hyperthermia with the localized and controlled release of a drug. This work was aimed to develop a composite based on magnetic nanoparticles covered with a poly (butylene succinate) matrix containing meloxicam. This drug is a non-steroidal anti-inflammatory drug which is useful in reducing pain and inflammation and, more recently; its antitumor activity is under investigation.2,3 The magnetite particles were synthesized by the Coprecipitation method.4,5 PBS was previously synthesized by the direct esterification procedure.6 The composite of magnetite and drug-coated with PBS was obtained by the emulsion – solvent evaporation technique. The yellow coloration of the particles prepared suggests that meloxicam was incorporated into the polymer matrix. FTIR results confirmed the obtaining of the composite. The resulting material was in the form of spherical particles with the mean diameter of 24.9μm. This composite has the potential for offering a combined effect of magnetohyperthermia and controlled drug release since they contain magnetic nanoparticles and meloxicam in their structure. Future studies will be performed to evaluate this approach.
Keywords: magnetic nanoparticles, magnetohyperthermia, drug release, poly (butylene succinate), meloxicam
NSAID, non-steroidal anti-inflammatory drug; PBS, poly (butylene succinate); MNPs, magnetic nanoparticles; MIH, magnetic induction hyperthermia; SAR, specific absorption rate
Controlled drug release systems can offer active agents into living organisms in a programmed manner. These release systems present several advantages. As the first one, they keep constant drug levels in the body, implying a lower drug content to produce the same effect as conventional systems. Besides that, they can preserve the drugs avoiding their destruction by the body. Finally, they can reduce the frequency of administration of the active agent, increasing patient comfort, and treatment efficacy.7 Among them, responsive controlled release systems represent a particular category because they release the drug only when receiving a stimulus which can be internal, from the body (such as temperature, pH, or enzymatic action) or external like a magnetic or electric field, ultrasound, and near-infrared light. The last ones are named on-off delivery systems, and they are especially advantageous because the drug can be released just at the desired moment.8
Magnetic nanoparticles (MNPs), especially the ones made of iron oxides, have excellent chemical stability, precise control over composition and structure and hence high efficiency in medical applications. However, MNPs have shallow zeta potential which is responsible for poor electrostatic repulsions and consequently a strong tendency to agglomerate.9 This process can occur by flocculation (small aggregates) or coagulation (denser aggregates). Therefore, for clinical use, these particles should be covered with biocompatible material to maintain colloidal stability but conserving their magnetic properties. Polymers are the most used materials to prepare magnetic nanocomposites which are more interesting than pure NPMs for biomedical applications.9
Poly (butylene succinate), PBS, is a crystalline thermoplastic aliphatic polyester with a melting point in the range of 90 to 120°C and a glass transition temperature between -45 and -10°C. This polymer has been receiving more attention because it can be obtained from renewable sources reducing the production costs and it is biodegradable and bioabsorbable.10
When these composites are placed under the action of an A.C. magnetic field, the vibration of the magnetic nanoparticles increases the local temperature (hyperthermia) and could promote the release of the drug included in the composite. This approach could be useful for designing combine therapies for several diseases such as cancer.11
Meloxicam is a non-steroidal anti-inflammatory drug (NSAID), which works by inhibiting cyclooxygenase2. NSAIDs are effective in reducing pain and inflammation and, more recently; Meloxicam antitumor activity is under investigation. However, the use of these medications is accompanied by several side effects, such as gastrointestinal bleeding, cardiovascular, and renal failure.2,3 So the use of a controlled release system can be more effective than systemic administration, reducing the side effects of the drugs.
This work was aimed to obtain an on-off delivery system based on PBS-covered magnetic nanoparticles containing meloxicam for combining hyperthermia and controlled release properties induced by the magnetic field.12
Materials
Ferric chloride hexahydrate (99.0%), potassium hydroxide (99.0%), ferrous sulphate heptahydrate (99%), 1,4-butanediol (99.3%), succinic acid (99.0%), sulfuric Acid (95-99%), ethyl alcohol, dichloromethane (99%), Titanium(IV) butoxide (Ti(OBu)4), poly (vinyl alcohol) 10,000g/mol, and Meloxicam were purchased from Sigma Aldrich.
Methods
The magnetite particles were synthesized by the Coprecipitation method. Ferric chloride hexahydrate (6.75g) and ferrous sulfate heptahydrate (6.95g) were dissolved in 30ml of deionized water. A solution of 1.12g of potassium hydroxide in 10ml of deionized water was then added, and the mixture was mechanically stirred for 30 minutes. The precipitate was washed to neutral pH, lyophilized and milled.
PBS was synthesized by the direct esterification method. Equimolar amounts of 1,4-butanediol and succinic acid and 0.1ml of sulfuric acid were used. One system was assembled using a heating plate under vacuum and nitrogen flow. At the outlet of the condenser, a flask under an ice bath was placed, and the trap was cooled using liquid nitrogen. After heating at 135°C for 6 hours, the catalyst (0.1% Ti(OBu)4) was added, and the temperature was gradually raised to 200°C. The reaction was kept at this temperature for 12 hours. The product of the synthesis was dissolved in dichloromethane and then mixed with excess ethanol in an ice bath to precipitate. This solid material was washed with ethanol and dried under vacuum for 24 hours.
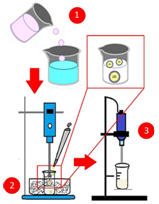
The composites of magnetite and drug-coated with PBS were obtained by the emulsion-evaporation method of the solvent, as shown in Figure 1. The magnetite (3.03vol%) and meloxicam (9.73vol%) were dispersed in 5ml of the PBS solution (10wt% of PBS in dichloromethane) using a high shear homogenizer (Ultra Turrax T-10, IKA, EUA). This dispersion was added to 50ml of a 0.5wt% polyvinyl alcohol in distilled water previously immersed in a bath of ice. The system was kept under agitation of the Ultra Turrax at 10 000rpm for 3minutes. The resulting emulsion was poured into a beaker with 400mL of 0.1% PVA solution and kept under stirring using a mechanical stirrer at 300rpm for 2 hours. The precipitate was washed three times with distilled water and lyophilized (L101, Liobras, Brazil).
The XRD analyses were performed at room temperature, using a multi-purpose X-ray diffraction in diffractometer Ultima IV from Rigaku Inc, model Miniflex with a radiation source that presented a potential difference of 40KV and an electric current of 20mA (Figure 2). The equipment target was a copper plate that produces CuKα radiation with a wavelength of 0.154nm. The scan was carried out at 2θ values from 15 to 4°C, with a goniometer step of 0.05°/min. The crystallite size (Lc) was calculated using the Scherrer's Equation (Equation 1):
(1)
In Eq. 1, Lc is the crystallite size, λ is the wavelength, β is the half-width and θ is the angle.
Materials were also studied using a Varian model 3100 FTIR Excalibur Series spectrophotometer (Japan). Samples were macerated with potassium bromide. Then, the FTIR spectra of the samples were recorded at room temperature using a resolution of 4cm-1. The Scanning Electron Microscopy was performed using a JEOL JSM-5610LV with acceleration voltage of 15kV. Magnetic force tests were performed using a homemade experimental setup, described elsewhere.5 The magnetic force (opposite to gravitational one) was calculated according to Equation 2.
(2)
Where Fm is the magnetic force, Δm is the apparent variation of mass in the presence of the magnetic field and g is the acceleration of gravity. As reference, the magnetic force of a cobalt (II) chloride hexahydride standard sample was calculated according this method and obtained result is equal to (0.11±0.01)mN at (458±3) Gauss.
Magnetic induction hyperthermia (MIH) tests were performed using an Ambrell EASYHEAT machine model L1. Samples, dispersed in distilled water (20mg/mL at 24°C), were thermally isolated using glass fiber textile. Soon afterward, each sample was inserted inside the machine’s coil. Samples were submitted to magnetic field produced using 770 A@222MHz for 900s. This condition produced alternating magnetic field equal 71.6 Gauss in the middle of the average diameter of the glasses. The total transferred power was equal to 105.7kW. The bulk temperature of the samples was measured before and after the induction heating tests. In addition, Specific Absorption Rate (SAR), which is used to evaluate the heat emission of a magnetic material when a resonant alternating magnetic field is applied, was calculated according Equation (3).13
ra (3)
Where Cnp is the specific heat of the nanoparticles (Cp=0.161cal.g-1.°C-1), CH2O is the specific heat of the water (Cp=1.000cal.g-1.°C-1), pH2O is the density of the water (1000mg/mL) and pnp is the mass concentration of the particles into the nanocomposite (2.2mg/mL).
The first indicative about the obtaining of the proposed material was its strong yellow coloration, proving the insertion of the drug into the polymer matrix.
Besides that, the X-ray diffractogram of pure maghemite presents peaks at 29.75°, 35.09° and 42.86°, which corresponding to (220), (311), and (440) crystalline planes from an orthorhombic crystal system.5 Crystallite size was calculated in accordance with Scherer’s equation (1), using the sharpest peak placed at 2θ equal to 35.09°, related to the (331) plane of the maghemite. The average particle size was equal to 19±2 nm.
Chemical identity of the samples was verified by Fourier transform infrared (FTIR) spectroscopy. The spectrum of the magnetic nanoparticles is shown in Figure 3A. This spectrum exhibits a broad signal around 3414cm-1 and a sharp uncompleted band at 587cm-1. These signals indicate the presence of -OH and Fe-O groups, respectively.14 The spectrum of the composite is shown in Figure 3B. This spectrum mainly shows the peaks of the PBS with a small signal at 3414cm-1, which is attributed to the magnetite. The reduced intensity of this signal is related to the small quantity of magnetic material into the composite. In turn, the PBS spectrum is shown in Figure 3C. This spectrum presents the characteristic absorption peaks of this polymer. They are the stretching vibration of the O-C-C at 1041cm-1, the stretching vibration of the C-O-C localized in the range of 1100-1300cm-1 and the carbonyl stretching centered at 1711 cm-1.6
SEM results are shown in Figure 4 & Figure 5. Magnetite nanoparticles presented an irregular shape with different sizes. On the other hand, the composites exhibited spherical shape and surface with small holes and some irregularities. The average diameter of the particles, calculated using the Image J software, was equal to 11±5μm.
Finally, the magnetic force of the composite was equal to 0.93mN/g, which was able to produce a SAR equal to 7.477W/g. Despite small, due to the low amount of magnetic nanoparticles used (3vol%), this is an encouraging result, since presented nanocomposite can produce heating under magnetic induction.
Spherical composites of PBS containing magnetite and meloxicam can be prepared by the emulsion-solvent evaporation technique. In that sense, it is expected that hyperthermia could be combined with the slow release of meloxicam. This combination is favorable due to the advantages that can offer but should be proved in future studies such as in vitro drug release alone or under a magnetic field.
Authors would like to thank to development institutions that have made this work possible: CNPq, CAPES, FINEP and FAPERJ.
The author declares no conflict of interest.

©2018 Moraes, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.