
Research Article Volume 1 Issue 3
Chemical reaction kinetics through dynamic mechanical analysis data
Mendoza Puente CI,1 Avalos Belmontes F,1
Regret for the inconvenience: we are taking measures to prevent fraudulent form submissions by extractors and page crawlers. Please type the correct Captcha word to see email ID.

Ramos deValle LF,2 Ortiz Cisneros JC1
1Faculty of Chemical Sciences, Universidad Autonoma de Coahuila, Mexico
2 Centro de Investigacion en Quimica Aplicada (CIQA), Mexico
Correspondence: Avalos Belmontes F, Faculty of Chemical Sciences, Universidad Autonoma de Coahuila, Blvd V. Carranza y Cardenas-Valdes, CP 25100 Saltillo, Coahuila, Mexico, Tel 52 (844) 4389830
Received: May 03, 2017 | Published: July 5, 2017
Citation: Puente MCI, Belmontes AF, Valle RLF, et al. Chemical reaction kinetics through dynamic mechanical analysis data. MOJ Poly Sci. 2017;1(3):109-111. DOI: 10.15406/mojps.2017.01.00015
Download PDF
Summary
The in situ study of chemical reactions kinetics has always been a great challenge. Here a modeling based on dynamic mechanical analysis (DMA) data of the natural rubber (NR) crosslinking reaction is presented and the order of reaction and reaction rate constant are determined.
Keywords: dynamic mechanical analysis, dicumyl peroxide, rheological, torque, correlation, viscosity, viscosimetric, polymerization, crosslinking
Abbreviations
DMA, dynamic mechanical analysis; NR, natural rubber; EPR, ethylene propylene rubber; MFI, melt flow index; PE, polyethylene; EPDM, ethylene propylene diene monomer
Introduction
The in situ determination of the chemical reaction kinetics in crosslinking or functionalization of polymer systems has been the subject of many recent studies. The decomposition of dicumyl peroxide during the crosslinking of NR was studied by Beyer.1 The effect of temperature and processing on the chemical kinetics of the functionalization of EPR (Ethylene Propylene Rubber) was studied by Greco et al.2
The incorporation of rheological studies to follow the advancement of a chemical reaction was carried out by Ryan,3 who implemented a Viscosimetric technique that implied a correlation between chemical changes and rheological changes. Modeling of the PE (polyethylene) crosslinking reaction kinetics based on data obtained via torque and capillary rheometry was studied by Ortiz-Cisneros4 in order to obtain the order of reaction and the reaction rate constant.
Understanding of the interactions between the reaction kinetics5 and the rheological characteristics is very important, especially, for example, if it is a polymerization or a crosslinking reaction, in which case could result in great viscosity and/or temperature increases. Thus, the setup of the rheometric data and its correlation and presentation in the form of simple reaction kinetics is of the utmost importance for the purpose of mathematical modeling.
Experimental
In any polymer system to undergo a crosslinking reaction, as the crosslinking density increases, the material moves from the rubbery to the glassy state. Therefore, the advancement of the chemical crosslinking reaction can be monitored through the continuous determination of the complex modulus
or its components, the elastic
and viscous
moduli, in an oscillatory rotational rheometer.
(1)
From the rubber elasticity theory, the complex modulus, determined at very low deformations, can be related to the average number of crosslinks “
”, as observed in equation (4). Additionally, if the rate of crosslinks formation
, is proportional to the number of crosslinks to be formed
; where
represents the total number of crosslinks at the end of the reaction and
represents the number of crosslinks at time t; and assuming that the kinetics of reaction follows the model of Kamal et al.5 it would result:
(2)
m and n are material constants to be determined by experimental data,
and
should be directly related to the rate constant of the crosslinking reactions. It has been determined that there exists a relation between the exponents m and n such that m+n=3 C Scalan etal.6 and it has also been proposed Francois Chambon et al.7 that for some systems
After integration and solving for
, it results:
(3)
If the reaction is treated as isothermic, then, the following equations apply:
(4)
(5)
Where
and
represent the complex modulus at a given reaction time and at the end of the crosslinking reaction. These two equations can be directly related to the crosslinking reaction kinetics.
The integration constant C can be evaluated by considering that at t=0,
.
This would give:
(6)
In the case of the material used for this work, a gel point value of 1.60556 * 103 Pa (150°C) was obtained, according to the technique mentioned by Tung and Dynes (Tung, C.-YM, Dynes, PJJ Appl Polym Sci 1982, 27, 569-574). When carrying out the experiment at 165°C, it is certain that it is above the gelation point of the material used. So that above the point of gelation
,
thus, it can be considered that
,
therefore:
(7)
It is important to mention that given the complexity of the equation, the nonlinear regression is performed as
Materials
The polymers used were: a metallocene isotactic polypropylene, MR 2001, (mPP) from Atofina, USA, with a molecular weight Mw of 185,000 and a MFI (Melt Flow Index) of 25 g/10min (230°C and 2.16 kg); and a IP-4640 EPDM (Ethylene Propylene Diene Monomer) (EPDM-55), with ca. 5 wt% of ethylidene norbornene as the diene monomer, with an ethylene content of 55 wt% and a Mooney Viscosity ML(1+4) at 125°C of 40, from DuPont-Dow Elastomers, USA.
* P stands for being in pellets forms
Results and discussion
We validated this model by monitoring the dynamic mechanical properties of the EPDM-55 formulation. We used an Anton Paar Physica rheometer with a cone and plate configuration at 165°C, within the linear viscoelastic region (Figure 1).
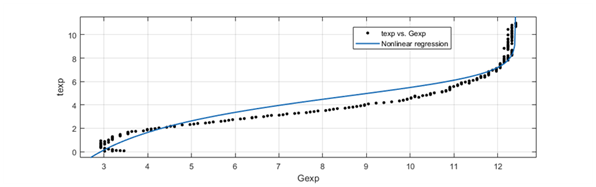
Figure 1 Shows the variation of G’ as the vulcanization (crosslinking reaction) of the sample proceeds, plus a nonlinear regression curve for the sample.
Data obtained from the nonlinear regression:
Coefficients (with 95% confidence bounds):
k=8.899e+04 (8.686e+04, 9.113e+04)
m=2.084 (2.066, 2.102)
The results of the linear regression can be corroborated by performing a dimensional analysis of equation (7), where the units of the kinetic constant are L2 mol-2 s-1, which corresponds to a third-order reaction, according to the proposal that m+n=3. The reaction is of third order since more than one reagent is involved in which the initial reactants maintain different concentrations. The model proposed here conforms to the experimental data with a high level of precision. This is in agreement with the experimental data of vulcanization.
Conclusion
The kinetic data of a crosslinking reaction can be obtained by dynamic mechanical analysis. This can be extrapolated to monitor many other polymeric reactions in which there is a change in the complex modulus coupled with the progress of the chemical reaction.
Acknowledgements
Conflict of interest
The author declares no conflict of interest.
References

©2017 Puente, et al. This is an open access article distributed under the terms of the,
which
permits unrestricted use, distribution, and build upon your work non-commercially.



