MOJ
eISSN: 2379-6383


Research Article Volume 8 Issue 5
1Center for Lipid Research, Key Laboratory of Molecular Biology for Infectious Diseases (Ministry of Education), Chongqing Medical University, China
2School of Public Health and Management, Research Center for Medicine and Social Development, Innovation Center for Social Risk Governance in Health, Chongqing Medical University, China
3The Chongqing Key Laboratory of Translational Medicine in Major Metabolic Diseases, The First Affiliated Hospital of Chongqing Medical University, China
4Department of Hepatobiliary Surgery, The Second Affiliated Hospital of Chongqing Medical University, China
Correspondence: Xue-Mei Lian, Center for Lipid Research, Key Laboratory of Molecular Biology for Infectious Diseases (Ministry of Education), Chongqing Medical School, No.1 Yixueyuan Rd, Yuzhong District, Chongqing 400016, China, Tel +86-23-68802030, Fax +86-23-68486780
Received: July 21, 2019 | Published: October 1, 2019
Citation: You-Qi-Le Wu, Xiao-Jing L, Guo-Chao Z, et al. Serum estradiol and testosterone levels in patients with lung cancer: A meta-analysis. MOJ Public Health. 2019;8(5):189-196. DOI: 10.15406/mojph.2019.08.00306
Sex hormones have been linked to cancer, although their relationship remains undefined. This study aimed to compare the serum blood levels of estradiol and testosterone among patients with lung cancer, those convalescing with benign pulmonary lesions, and healthy individuals. An electronic search was conducted for relevant studies that were published up to May 2019 and provided the mean and standard deviation of the serum blood levels of sex hormones. A random–effect model was utilized to achieve significant heterogeneity from the published data. Nineteen studies involving 961 patients with lung cancer, 262patients with benign pulmonary lesion, and 676 healthy individuals were analyzed. The meta–analysis showed that estradiol serum blood levels were higher in patients with lung cancer than in those with pulmonary lesion (standard mean difference [SMD]=4.58, 95% confidence interval [CI] [–0.61, 9.77], P=0.08) and healthy individuals (SMD=5.65, 95% CI [1.21, 10.08], P=0.01). By contrast, the testosterone serum blood levels were lower in patients with lung cancer than in those with pulmonary lesion (SMD=–0.97, 95% CI [–1.60, –0.33], P< 0.01) and healthy individuals (SMD=–1.05, 95%CI [–1.50, –0.59], P<0.01). Subgroup analysis showed that the above–mentioned observations were more pronounced in men than in women (P for interaction <0.05). In Chinese population, higher estradiol serum blood levels but lower testosterone serum blood levels were found in patients with lung cancer compared with those of patients with benign lung lesion and healthy individuals. Our findings provided epidemiological support for the role of sex hormones in the pathogenesis of lung cancer. The generalizability of these findings to other populations must be further studied.
Keywords estradiol, testosterone, lung cancer, meta–analysis, Chinese population
Lung cancer is the most frequently diagnosed cancer and the most common cause of cancer death worldwide, particularly in China with a total of 782,000 new cancer cases and 626,000 cancer deaths in 2014.1 the high incidence of lung cancer is affected by risk factors, such as smoking as the primary and gender. Women smokers seem to have greater risk of developing lung cancer compared with their nonsmoking counterparts.2 among sex steroids, estradiol and testosterone are the major contributors of lung cancer pathogenesis. Estrogen plays an important role in the progression of lung cancer through genomic or non–genomic pathways; furthermore, certain proportions of estrogens in situ are produced through aromatase from circulating precursor testosterone.3 Although serum estrogen is substantially associated with a poor lung cancer survival rate,4 data on the relationship between circulating steroid hormone levels and lung cancer status are limited. In this study, we performed a meta–analysis to compare the estradiol and testosterone serum blood levels of patients with lung cancer with those having benign pulmonary lesions and healthy individuals.
Literature search and inclusion and exclusion criteria
We performed an electronic search of the PubMed, Cochrane Library, China National Knowledge Internet database, VIP, WANG FANG MED, and Sino Med from the date of publication inception to May 2019 with restriction to the English and Chinese languages. We used the following keywords for searching: “serum sex hormones,” “serum estradiol,” “serum testosterone,” “lung cancer,” “lung carcinoma”, and “lung neoplasms”. In addition, we reviewed reference lists from retrieved articles to identify any potentially relevant studies. We did not contact the authors of the included studies to obtain additional information.
Inclusion criteria for eligible studies were as follow: First, the studies must be domestic or foreign literature published on the serum blood levels of sex hormones and lung cancer with the reference languages limited to English and Chinese. Second, the patients must be diagnosed with lung cancer. Third, case control studies must compare the estradiol or testosterone serum blood levels of patients with lung cancer with those with benign pulmonary lesions or healthy individuals. In addition, the studies must provide the mean and standard deviation of serum sex hormones. We excluded studies that measured the serum sex hormones of patients after surgery or chemoradiotherapy.
Data extraction and quality assessment
Two reviewers (Y.W and X.L) utilized the Newcastle–Ottawa scale (NOS) to independently perform quality assessment for the included studies. This scale consists of eight items categorized into three aspects (i.e., selection, comparability, and outcomes). An individual study may earn a maximum of nine stars after the evaluation of its three aspects. In this review, high–quality studies were defined as those earning≥7 stars. Any discrepancies regarding the results of quality assessment were handled through discussion.
Statistical analysis
With regard to the different detection methods and measurement units of serum sex hormones among the included studies, standardized mean difference (SMD) was utilized to reveal between–group differences. Random–effect and fixed–effect models were undertaken to calculate the weight of each effect measure for data with and without heterogeneity, respectively. The characteristics of the included studies involved the assessment of clinical and methodological heterogeneity utilizing Chi2 and I2 statistics. Substantial inconsistency across the studies was set when the P–value was <0.1, and I2 was >50%. We performed predefined subgroup analyses to investigate whether our pooled results were modified. Sensitivity analyses were performed by applying our various exclusion criteria, ignoring a single study in turn, and repeating meta–analysis through a fixed–effect model to identify possible sources of heterogeneity and to evaluate the stability of pooled results.
Publication bias was assessed with the Begg’s and Egger’s methods. Data synthesis and analysis were performed by utilizing the RevMan5.3 software. Statistical significance was set at a probability level of <0.05 under the two–sided test unless otherwise specified.
Literature research
The specific process of our literature search is shown in Figure 1. Seventeen potential studies were identified after full–text screening. Two additional works were selected after the reference screening. Altogether, a total of 19 studies were included in our meta–analysis.
Study characteristics
For the 19 included studies, 961 subjects were in the lung cancer groups, 676 ones in the healthy control groups, and 262 ones in the benign pulmonary lesions groups. The participants of 9 studies consisted of both men and women, whereas 9 studies included only men, and 1 study included only women. The basic characteristics of the included literature are detailed in Table 1.
|
Author |
Year |
Gender |
Number of lung cancer cases |
Number of healthy cases |
Number of benign lesions cases |
Detection method |
Test content |
Quality evaluation (stars) |
|
Wang et al.5 |
1994 |
M, F |
21 |
40 |
/ |
RIA |
E2, T |
6 |
|
Luo et al.6 |
1994 |
M |
47 |
20 |
/ |
RIA |
E2 |
6 |
|
Chen et al.7 |
1996 |
M, F |
50 |
20 |
/ |
RIA |
E2, T |
6 |
|
Shao et al.8 |
1998 |
M |
68 |
42 |
/ |
RIA |
E2, T |
6 |
|
Li et al.9 |
1998 |
F |
22 |
25 |
/ |
RIA |
E2, T |
6 |
|
Chen et al.10 |
1999 |
M, F |
53 |
53 |
53 |
RIA |
E2, T |
7 |
|
Zhou et al.11 |
2001 |
M |
70 |
30 |
30 |
IEMA |
E2, T |
6 |
|
Zhou et al.12 |
2002 |
M |
25 |
30 |
8 |
IEMA |
E2, T |
6 |
|
Hao et al.13 |
2004 |
M, F |
60 |
30 |
/ |
CLIA |
E2, T |
7 |
|
Chen et al.14 |
2005 |
M |
62 |
20 |
24 |
RIA |
E2 |
6 |
|
Su et al.15 |
2007 |
M |
27 |
34 |
19 |
CLIA |
E2, T |
7 |
|
Gu et al.16 |
2010 |
M, F |
27 |
30 |
30 |
CLIA |
T |
7 |
|
Xu et al.17 |
2011 |
M, F |
54 |
10 |
/ |
RIA |
E2, T |
7 |
|
Gu et al.18 |
2014 |
M, F |
47 |
32 |
45 |
ELISA |
E2, T |
7 |
|
Zhang et al.19 |
2015 |
M, F |
60 |
60 |
/ |
CLIA |
E2 |
7 |
|
Zheng et al.20 |
2015 |
M,F |
48 |
40 |
/ |
CLIA |
E2 |
8 |
|
Zhang et al.21 |
2016 |
M |
42 |
/ |
23 |
ELISA |
E2, T |
7 |
|
Gao et al.22 |
2017 |
M, F |
40 |
40 |
40 |
CLIA |
E2 |
7 |
|
Zhang et al.23 |
2017 |
M |
110 |
80 |
80 |
CLIA |
E2, T |
7 |
Table 1 Basic characteristic of the included studies
Abbreviations: M, male; F, female; RIA, radioimmunoassay; IEMA, immuno enzymometric assay; ELISA, enzyme-linked immunosorbent assay; CLIA, chemiluminescent immunoassay; E2, estradiol; T, testosterone.
Meta-analysis
Estradiol and lung cancer: Our pooled results showed that the estradiol serum blood levels were higher in the lung cancer group than in the healthy control group (SMD=5.65, 95% CI [1.21, 10.08], P=0.01, Figure 2). Similarly, the aforementioned observation was also detected when we compared the lung cancer group with the benign pulmonary lesion group (SMD=4.58, 95%CI [–0.61, 9.77]) with marginal significance (P=0.08, Figure 3).
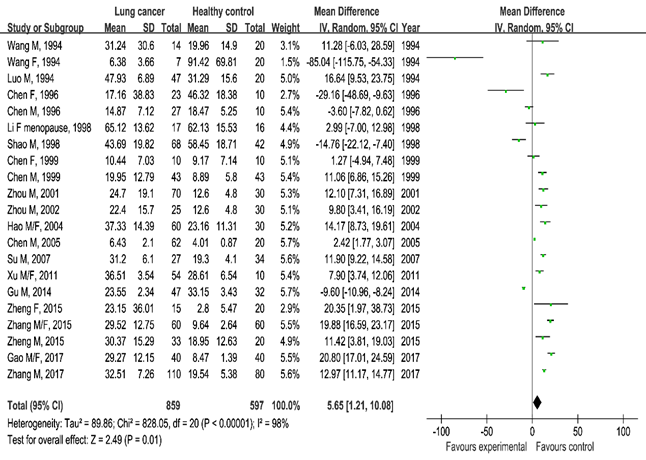
Figure 2 Meta-analysis of serum estradiol levels in the lung cancer group and healthy control group.
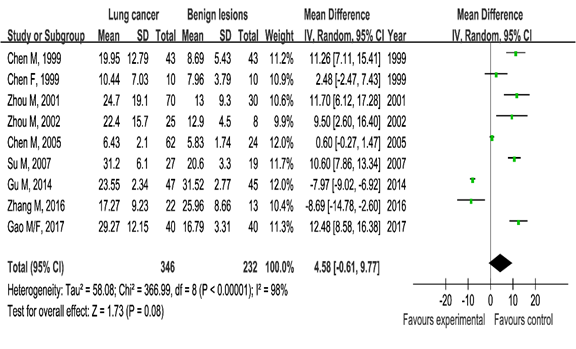
Figure 3 Meta-analysis of serum estradiol levels in the lung cancer group and benign lung lesion group.
Testosterone and lung cancer: The lung cancer group showed lower testosterone serum blood levels than the healthy control group (SMD=–1.05, 95%CI (–1.50, –0.59), P<0.00001) and the benign lung lesion group ((SMD=–0.97, 95%CI (–1.60, 0.33), P=0.003). These statistically significance results showed the correlation between serum testosterone and lung cancer (Figures 4 & 5).
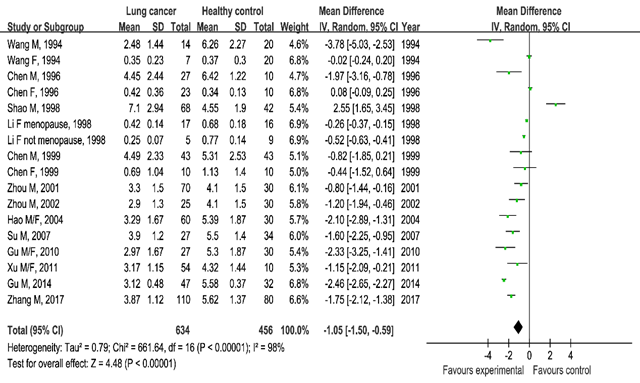
Figure 4 Meta-analysis of serum testosterone levels in the lung cancer group and healthy control group.
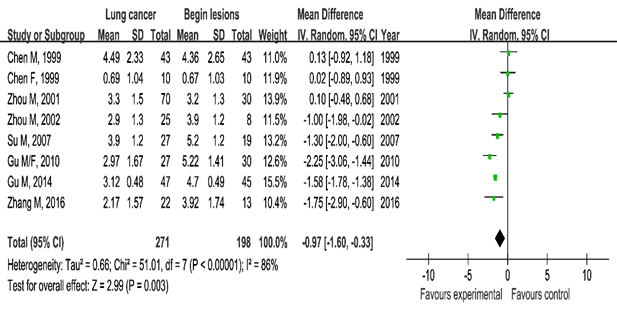
Figure 5 Meta-analysis of serum testosterone levels in the lung cancer group and benign lung lesions.
Subgroup analysis: We conducted pre–specified subgroup analyses to determine whether the observed association between the serum sex hormones levels and lung cancer was modified by detection methods, sex, number of cases, NOS stars and publication year, and adjustment for confounders. P interaction for the difference between subgroups was calculated. The results showed that different detection methods may greatly contribute to heterogeneity. In addition, the results of testosterone were consistent in both of the healthy controls and benign lesion group, whereas estradiol results were less stable (Figures 6 & 7).
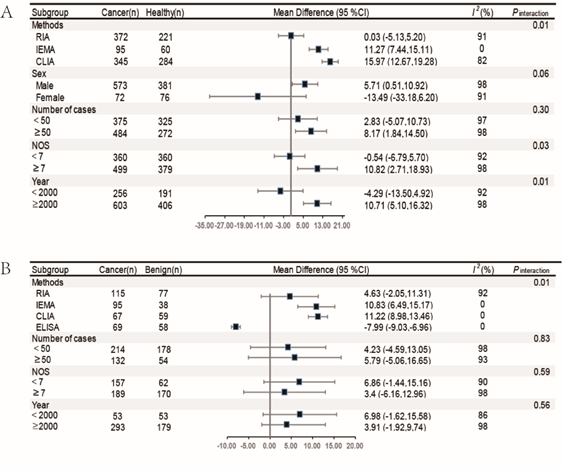
Figure 6 The subgroup analysis of serum estradiol levels in lung cancer group and healthy controls and benign lesions.
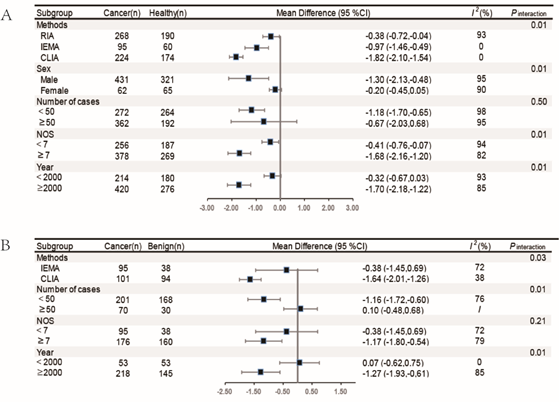
Figure 7 The subgroup analysis of serum testosterone levels in lung cancer group and healthy controls and benign lesions.
Publication bias
We did not find evidence of publication bias for any association with Begg’s and Egger’s tests (P>0.05).
The role of estrogenic steroid hormones in the etiology of lung cancer is not entirely clear, although lung cancer is widely accepted as a hormone–related cancer.24 This article reviewed 19 studies that analyzed 961 patients with lung cancer, 262 patients with benign pulmonary lesion, and 676 healthy individuals. The meta–analysis showed that the estradiol serum blood levels were higher in patients with lung cancer than in those with pulmonary lesion and healthy individuals. By contrast, testosterone serum blood levels were lower in patients with lung cancer than those in patients with pulmonary lesion and healthy individuals.
Estrogen is an important steroid hormone that has been postulated as a contributor in the development and progression of many cancer types. However, the results are inconsistent in the literature.25–27 Zhang et al.25 found that female patients with esophageal squamous cell carcinoma (ESCC) and a higher estradiol serum blood level have a favorable survival than those with low serum levels, indicating the protective effect of serum estradiol on ESCC.25 Patients with breast cancer in the Geneva Cancer Registry who utilized antiestrogens showed significantly reduced lung cancer mortality.28 A high level of circulating estrogen can increase the risk of postmenopausal breast cancer, especially estrogen receptor positive tumors.29 The detection of estradiol serum blood levels may also contribute to the clinical early diagnosis and treatment of lung cancer. Serum estrogen is significantly associated with poor lung cancer survival, and specific genotypes affecting serum estrogen and tumor ER-α expression levels are associated with lung cancer prognosis. Existing studies have shown inconsistency about the correlations between serum estradiol and lung cancer status. In our subgroup meta-analysis, the statistically significant increase in the serum estradiol levels in lung cancer groups was observed in males but not in females. Given the lack of information related to reproductive factors (e.g., menopausal status, age at menarche, oral contraceptive use, postmenopausal hormone use), the association between serum estradiol and lung cancer in female groups still does not have a definitive conclusion. Well-designed further studies are needed to obtain conclusive results.
The association between androgen represented by testosterone and cancer has been less explored. Cohort studies showed that elevated testosterone serum blood levels are associated with an increased risk of early cancer death. High testosterone serum blood levels may be associated with lung cancer and incidental prostate cancer.30, 31 The present study showed that serum testosterone levels were lower in the lung cancer population than in the control groups. Aromatase, a rate-limiting enzyme that converts androgen into estrogen in lung tissues, can promote the conversion of testosterone to estradiol and may be the cause of decreased testosterone serum blood levels in patients with lung cancer. Unfortunately, most of the included reports do not mention the level of aromatase in lung tissues.
This study has limitations. First, the results may be suitable only for the Chinese population because no articles of foreign literature were retrieved. Second, although subgroup analysis revealed that the detection method for hormone is the major source of heterogeneity, a large part of the heterogeneity remains to be analyzed. Third, confounders, such as smoking status, female reproductive factors, classification and stage of lung cancer, were not addressed in most of the recruited studies. Fourth, the sample size of the study on the correlation between serum estradiol and lung cancer in females was not sufficiently large. Therefore, further stratified studies are needed to reveal the association.
Patients with lung cancer show higher serum estradiol levels but lower serum testosterone levels compared with those having benign lung lesion and healthy individuals. This result suggests the serum blood levels of sex hormones as a biomarker for the diagnosis of lung cancer. Our findings provide some epidemiological support for the role of sex hormones in the pathogenesis of lung cancer. The generalizability of these findings to other populations must be further studied.
This work was supported by the Natural Science Foundation of Chongqing Yuzhong District (No. 20170402).
None.
Author declares that there is no conflict of interest.

©2019 You-Qi-Le, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.