MOJ
eISSN: 2379-6383


Research Article Volume 12 Issue 1
1Department of Zoology, GPGC Mardan KPK, Pakistan
2Professor, PMC, Peshawar, KPK, Pakistan
3Department of Zoology, Hazara University Mansehra KPK, Pakistan
4Department of Microbiology, Abasyn University Peshawar KPK, Pakistan
5Department of Microbiology, Hazara University Mansehra KPK, Pakistan
6Department of Zoology, AWKUM University Mardan KPK, Pakistan
7Department of Zoology, Islamia College University Peshawar KPK, Pakistan
8Department of Microbiology, KUST, Kohat KPK, Pakistan
Correspondence: Manzar Khan, Department of Zoology, Hazara University Mansehra, KpK Pakistan-21300, Tel +92 305-1701035
Received: October 27, 2022 | Published: February 21, 2023
Citation: Ali S, Ali B, Khan BB, et al. Sero-prevalence of hepatitis-c virus among blood donors in northern Pakistan. MOJ Public Health. 2023;12(1):37-41. DOI: 10.15406/mojph.2023.12.00407
HCV is a hepatotropic single strained positive sense RNA virus belongs to family Flaviviridae. HCV is worldwide distributed virus affects about 185 million peoples as a result it causes 500,000 deaths per year. In Pakistan 5-6% of individuals are infected with HCV while in blood donors this ratio varies from 1.05-3.24 in different region of Pakistan. In Peshawar sero-prevalence of HCV was 4 percent. This study focuses on the actual status of HCV among blood donors at district Mardan and to compare the findings with previous study and shows the differences. The data were collected in MMC-MTI Mardan from January 2019 to May 2019. Through CMIA (by architect 1000i) methods the quantitative detection of anti-HCV antibodies takes place and 625 samples diagnosed through EIA methods, in which 9 were positive to HCV antibodies. Overall sero-prevalence of HCV positive donors is 1.44% and high prevalence were founded in the male which was (1.45%), rural area (1.95%), illiterate donors (2.08%), married donors (2.20%), donors belong to lower class economic status (2.21%), 1st time donors (1.72%) and age group 38-47 (3.64%) were positive to HCV. The risk factors of HCV transmission are IVD use, unscreened blood transfusion, contaminated surgical instruments and shaving in barber shop. The findings of this study show that the sero-prevalence of HCV increased 0.24% from 2018 among blood donors at district Mardan. It was recommended that more studies required to explore the seroprevalance of HCV in other cities of Pakistan.
Keywords: hepatitis, blood donors, prevalence, transfusion, hepatitis-c virus, sero-prevalence
Hepatitis C Virus (HCV) also called silent killer because many peoples have no idea about HCV even HCV present in their body, means show no sign and symptoms or asymptomatic.1 Hepatitis is a term mainly used for the inflammation of liver caused by type of viruses which have specificity and affinity for liver cell.2 HCV is the main pathogen of human which causes chronic and acute infection.3 Hepatitis C virus belongs to family Flaviviridae, Hepatitis C is a viral infectious disease of liver caused by HCV and the genome of HCV consists of positive sense single-stranded RNA (9600 nucleotides) discovered in 1989.4,5 HCV is worldwide distributed virus affects about 185 million peoples as a result it causes 500,000 deaths per year.6,7 New cases of HCV infection occur from 3-4 million yearly in the entire world.8
The mostly effected area of the world is North Africa and Middle East that have high frequency of HCV. Almost 15 million peoples are prey of HCV in Middle East and North Africa (MENA). In Pakistan more than 10 million individuals are involved in HCV [15, 16]. HCV prevalence in general population of Pakistan varies from 4.32-11.14% while 1.05-3.24% prevalence founds in blood donors. About 11 million peoples in Pakistan are involved in HCV infection which is 5-6% part of Pakistan whole population.9,10 Unluckily Pakistan is counted in high prevalence countries of HCV with probable prevalence of 6.7% and new study recommended it increase to 8.64% in Pakistan. The cause of this high prevalence of HCV in Pakistan is un-hygienic condition, low level of education and low socioeconomic rate.11,12 Approximately more than 110 million unit of blood are collected annually in whole world and every transfusion of blood leads to a risk of transmitting blood-borne pathogens, including HCV.13 Among voluntarily blood donors (VBD) in china ratio of HCV is 1.7%. Recently studies showed that the ratio of HCV in VBD varies from 0.25% to 0.86% in different area of China. In Iran and Brazil the prevalence of HCV in VBD is 0.13% and 0.19% respectively. In Nigeria the rate of HCV infection varies from 1.4 to 5.4% among blood donors in deferent cities.14,15
HCV is blood-borne pathogen that leads to cirrhosis, fibrosis, HCC and liver transplantation but some time become fatal and effects the economy of country.16 Acute HCV means appears of sign and symptoms or HCV antibodies, it may occur after 6 months of infections.17 In 1975 the presence of HCV was completely realized when large number cases of transfusion-associated hepatitis were not any one of hepatitis-A and hepatitis-B.18 Hepatitis C virus (HCV) is involve in serious liver disease and also responsible for blood-born infection. HCV was recognized for the first time in the serum of a man which have not any one of hepatitis A or B in 1989.19 HCV have mainly 7 different genotype (e.g., 1a, 1b, 2a, 2b, 3a, 3b, 3c) and they also sub classified into 80 genotypes, this large number of genotypes of HCV is due to HCV polymerase that have no ability of proofreading and also have reverse transcriptase so the rate of mutation is high in each cycle.20,21 The distribution of HCV genotypes varies globally. Genotypes 1-3 are widely distributed in the world.22 Genotypes 1 of HCV is the most prevalent of all genotypes and responsible for 46% of HCV causing agents, genotypes 3 cause 22% of HCV infection and genotypes 2 & 4 cause 13% of HCV infection.23 Hepatitis can be caused by Immune cells in the body attacking the liver. Infections from viruses (such as hepatitis C), bacteria24 or parasites. Liver damage from alcohol or poison. Medicines, such as an overdose of acetaminophen and through Fatty liver.9
Transmission of HCV commonly occurs with contact or ingestion of HCV containing blood, blood products transfusion; inter venous drug using, hemodialysis and transplantation of organs. More ever rarely transmission also occurs due to sexual intercourse.25,26 Normally also transmitted by blood-to-blood contacts even injuries with contaminated equipment and sharing of needle, From mother to infant transmission also possible.10,27 HCV transmission also occurs by unsterilized medical instrument, use of hazards equipment for piercing of nose ears and shaving. HCV cross placenta so from infected mother HCV also transfer to fetus through placenta.28 The risk of HCV transmission through blood transfusion decreased in developed countries whiles the countries which have limited resources due to reuse of syringes and needles transmission of HCV take place.29 Screening of blood donors is necessary to stop further spread of viral infections because the prevalence of blood borne viral infection is increasing globally day by day.30,31 TTI defined as any disorder that transfer from one individual to another individual by parental direction of blood or its products.32 The donation of blood save the life of blood recipients patient but there are some transfusion transmissible infection (TTI) which also transfer from blood donor to recipients like hepatitis B virus (HBV), HCV, human immunodeficiency virus (HIV) and syphilis. Because of these risk factors WHO suggested that all donors must be screened before donation (Figure 1).33,34
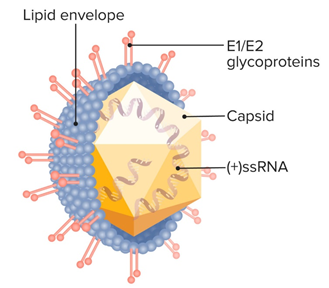
Figure 1 Structure of Hepatitis-C Virus.14
Occult HCV Infection (OCI) is the condition of chronic HCV in which HCV-RNA present in hepatocyte (liver cells) or peripheral blood mononuclear cells (PBMCs) but the detection of HCV-RNA and anti-HCV antibody does not occur from serum or from plasma.15,21 To find HCV-RNA in OCI condition in liver cell the diagnostic method must be liver biopsy. Due to OCI the minimum changes occur in liver cell.35 For the 1st time OCI was reported by Pham in anti-HCV positive individual with normal liver enzymes and individual with SVR because of interferon (IFN). In the same year, Castillo was detected HCV RNA presence in anti-HCV negative individuals with high liver enzymes.36 A new type of CHC conformed in 2004 by Castillo and their colleagues. They investigated 100 patients and results obtained from serum for HCV antibodies and HCV-RNA was negative but the HCV-RNA detected in hepatocytes through revers-transcription polymerase chain reaction (RT-PCR). OCI is a condition in which a patient shows negative serological results but RNA quantification is positive.35
A study conducted in Leady Reading Hospital Peshawar, according to this study HCV prevalence founded 4% in healthy blood donors.37 HCV prevalence is much lower in healthy VBDs as compared to commercial donors. HCV prevalence among blood donors in Pakistan were reported 3.78%.38,27 The sero-prevalence of HCV in healthy blood donors in Iran in the year 1994 were 0.25% and in the period of 2004-2007 the study conducted in Iran that were shown the prevalence of HCV 0.13%. Among blood donors, from 0.4% to 19.2% prevalence of HCV is changed worldwide.39 There is no HCV prevalence studies in blood donors in Mardan population have been conducted and the true burden of infection is unknown. So this study focuses on the actual status of HCV among blood donors at district Mardan and to compare the findings with previous study and shows the differences. Therefore, this study focused on the Sero-Prevalence of Hepatitis-C Virus among Blood Donors in Mardan KPK Pakistan that can be helpful for researchers, health workers and community. This study uncover the critical areas of Sero-Prevalence of Hepatitis-C Virus among Blood Donors that many researchers were not able to explore.
Materials that used were sterile. The materials were used in experiment were: Blood Bag, Disposable, Gloves, Mask, Laboratory Coat, Gel Tube, Centrifuge Machine, Anti-Sera, Slides, Freezer, Scissor, Shaker machine, Micro Pipits and Yellow tips. The data were collected in MMC-MTI Mardan from January 2019 to May 2019. The data were collected through direct interviewee questionnaire and the main question were asked from the donors were about their age, gender, area, education, type of donation. After that 3-5ml blood were taken in gel tube for the further process. Next, we were finding blood group. The gel tubes having sample of blood donors’ centrifuge for 5-10 minutes at speed of 4000 runs per minute (RPM). The centrifuged gel tubes were ready for screening now. The serum was kept in serum cup. CMIA is the modern types of enzyme immunoassay (EIA) technique.40
Enzyme immunoassay
EIA is the most common diagnostic laboratory techniques used for the detection of anti-HCV antibody. Now a day 3rd generation of EIA is available (Li et al., 2015). HCV is not detected in serum by strip, EIA and ELISA serological method at initial stage because anti-HCV antibodies established after 45 days of infection Arshad et al.,9 The donation of blood screen for Anti-HCV by EIAs has decrease the risk of Transfusion-Transmitted Infection (TTI). CMIA is used to prevent HCV transmission to recipient of blood, blood products, tissue, cell organs etc. Architect is based on chemiluminescent (CL) micro-particle immunoassay (CMIA). Architect Anti-HCV assay is CMIA that were used for qualitative detection of antibodies to HCV in serum or plasma sample. Architect Anti-HCV has been designed to point out antibodies to non-structural and structural protein of HCV genome. The automation software was installed to Architect. After 40-50 minutes single test process was completed for HCV and other viral analysis like HBV, HIV and VDRL.
The data were collected in blood bank and diagnosed for anti-HCV antibodies in serological department of laboratory of MMC-MTI. Total sample size was 625 and was diagnosed through CMIA method by 1000i architect. Overall sero-prevalence was (1.44 %). Previously,41 conducted study in Baluchistan and reported HCV in blood donors by ELISA test which is 20.8% positive higher than present study. On other hand slightly similar result to 109/5517 (1.9%) blood donors were positive for HCV while in Islamabad with HCV prevalence in blood donors was 8.34% higher than present study because growing pool of asymptomatic carriers.42,43 This study suggested sero-prevalence in male population was (1.45%) while females all were negative (06) donors because of cultural restriction, dormancy of male and more respect to female in the society and sacrificing behavior of male whereas,44 were reported HCV positive in males 10% while 2% in female donors in Sargodha that is higher than current study because sharing of nail cutter, shaving in barber shop, sharing of shaving instruments and I/V injections used. Another study reported HCV positive donors were 0.46% in china which is lower than present study.14
The results from the present study suggested that among the observed urban population of Mardan sero-prevalence of HCV was 1.95%. It was observed that high sero-prevalence of HCV anti-bodies found in illiterate donors (2.08%) while45 diagnosed blood donors for HCV in Abeokuta, Nigeria in educated people and an uneducated people lower than present study. Married donors were diagnosed 2.20% positive for anti-bodies. Donors belong to lower class economic status was 2.21% positive, whereas study conducted by Kamran et al.,46 that reported 3.43% HCV in married donors and 0.89% in single donor that is higher than present study because the use of unsterilized surgical instruments especially in dental clinic. With respect to present study sero-prevalence of HCV in 1st time donors and repeated donors 1.72% and 1.19% respectively, previously47 in Veracruz, Mexico described that HCV in 1st time donors were 0.57% and repeated donors were shown 0.31% prevalence of HCV lower than current research. Another study conducted by Chama et al.,48 in Lusaka, Zambia, in which sero-prevalence of HCV in 1st time donors and repeated donors were observed 0.5% and 0.6% respectively (Figure 2).
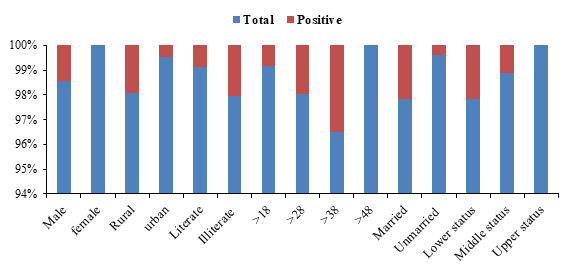
Figure 2 Shows the gender, area, and education status, and age, marital and economic status of samples.
The results from study advised that volunteer donors all were negative because they all were young and educated while exchangeable donors diagnosed 1.9% sero-positive for anti-HCV antibodies while49 in Kathmandu, Nepal, reported HCV prevalence in volunteer donors was 0.7% higher than present study because of present investigation shows that volunteer donors are healthy, young, and most are educated. In contrast49 describe HCV prevalence in replaceable donors was reported 0.4% lower than present study. The sero-prevalence of HCV in different age groups was found that HCV was maximum (3.64%) in age group 38-47 years while above 48 years donors all were negative because they were healthy and very few (10) donors belongs to this group. Previously,50 concluded high prevalence in age group of 26-35 while no positive donor were founded in age group 46-55 because of few donors and similar to current study. Another study conducted by51 in Zaria, Nigeria which have high prevalence founded in age group of 20-24 and 25-29 and low prevalence of HCV founded in age group of 30-34 that is higher than present study it may be inter veins drug use and unsafe blood transfusion.
The new investigation suggested sero-prevalence of HCV anti-bodies in blood donors with respect to their blood groups, the maximum sero-prevalence were found in O-negative (6.25%), followed by A-negative (5.55%) and then B-positive (2,6%) while remain all were negative while The study conducted in Debre tabor town; Ethiopia describe that Rh+ve donors were 6612 (91%) and 578 (8.0%) were Rh-ve in which 41 were positive to HCV antibodies in Rh+ve and 8 were positive in Rh-ve type donors with total percentage of 0.57% and 0.11% respectively which is lower than present study might be because of the actual differences in population risks or severity and efficiency of the procedures followed of blood gathering process (Figure 3).52-54
The results of present study showed that there is prevalence of HCV founds in blood donors visiting to Mardan Medical Complex. This prevalence is high then developed countries because of un-awareness and less health facilities. The blood donors were mostly male and the contribution of female for donation of blood was very less because of cultural restriction and sacrificing behavior of males. According to present study outcomes it is concluded that infection of HCV in healthy blood donors due to certain risk factors like contaminated surgical instruments especially in dental clinic, shaving in barber shop, direct contact with blood and its products. The Overall sero-prevalence was (1.44 %). This study suggested sero-prevalence in male population was (1.45%) while females all were negative (06) donors because of cultural restriction, dormancy of male and more respect to female in the society and sacrificing behavior of male. The urban population of Mardan sero-prevalence of HCV was 1.95%. It is concluded that 0.24% increased occurred in HCV sero-prevalence among blood donors. Owing to its simplicity, low cost, and rapidity, we would recommend this study design as a model for sero-prevalence studies for other diseases in other cities of KPK, or for HCV sero-prevalence studies elsewhere in Pakistan.
We are thankful to all the individuals that participated in this research study. We are glad to the pathology department of Mardan medical complex and Mardan teaching institute Mardan (MMC-MTI) I would like to express my deep and sincere gratitude to my supervisor Mr. Basit Ali Lecturer in Department of Zoology, Government Post graduate College Mardan. I am thankful to him, for his valuable advice and friendly help. Last but not the least I owe my loving thanks to my parents that support me on every step of life.
The authors declare that there is no conflict of interest.

©2023 Ali, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.