MOJ
eISSN: 2379-6383


Research Article Volume 13 Issue 3
1Programa de Posgrado en Ciencias Ambientales, Facultad de Ciencias, Universidad de la República, Uruguay
2Unidad de Salud Pública Veterinaria, Facultad de Veterinaria, Universidad de la República, Uruguay
3Unidad de Zoonosis y Vectores, Ministerio de Salud Pública, Uruguay
Correspondence: Gustavo J Nagy, Programa de Posgrado en Ciencias Ambientales, Facultad de Ciencias, Universidad de la República, Iguá 4225, Montevideo, Uruguay, Tel (598) 2 5258618-21
Received: November 04, 2024 | Published: November 18, 2024
Citation: Vidal B,Verger L, Nagy GJ. Emergent and re-emergent zoonotic infectious diseases in Uruguay (2004-2024). Part II: Arboviral diseases. MOJ Public Health. 2024;13(3):178-185. DOI: 10.15406/mojph.2024.13.00458
In a previous communication, the authors followed a One Health approach to analyse human, animal, and socio-environmental and climatic changes affecting the emergence and re-emergence of zoonotic infectious diseases (ZID) in recent decades in Uruguay. Based on Uruguayan’s WOAH reports and the zoonotic reports from the Ministry of Agriculture and Livestock and the Ministry of Public Health, the focus was on Rabies, Leishmaniasis, Avian Influenza, and Western Equine Encephalitis. This research article briefly summarises these results, adding the analysis of the available data on Arboviral diseases transmitted by Aedes aegypti, Dengue and Chikungunya, based on the reports from the Ministry of Public Health and the Pan-American Health Organization. We analyse their recent outbreaks and distribution, highlighting the potential impact of our findings. Finally, we deepen our previous comparative analysis of the monitoring sources, which, as we reported, show some differences and methodological gaps requiring improvement and verification, adding the recent autochthonous Dengue and Chikungunya reports since 2023.
Keywords: zoonoses, climate, arboviral diseases, public health, monitoring, policies
Uruguay has been subject to various productive and socio-environmental-climatic alterations over the last few decades. Emerging and re-emerging zoonotic diseases (e.g., Rabies, Leishmaniasis, Avian Influenza, Western Equine Encephalitis) are reported by the Ministry of Agriculture, Livestock and Fisheries (MGAP) and the Ministry of Public Health (MSP), and then are sent to world organisations as the World Organisation of Animal Health (WOAH) in the case of MGAP or the Pan American Health Organisation (PAHO) by the MSP. Zoonoses are managed separately, as the occurrence of Arboviral diseases transmitted by Aedes aegypti (Dengue and Chikungunya) is reported by the MSP. However, the MGAP reports most of the zoonoses.
This research article deals with the emergence and re-emergence of zoonotic infectious diseases (ZIDs) in Uruguay over 2007-2024 from a One Health (OH) perspective, which optimises interdependent human, animal, and ecosystem health.1 Here, we build upon a previous communication1 that focused on Bat Rabies, Visceral Leishmaniasis, Avian Influenza, and Western Equine Encephalitis, summarise the main results, and extend the analysis to the recent outbreaks of Arboviral zoonotic infectious diseases affecting the country, namely Dengue and Chikungunya. Besides, this research article redraws and extends a table comparing monitoring and reporting infectious diseases, some differences, and contributions to improving zoonotic public health reporting and policies.2
Emerging zoonotic infections are linked to socio-environmental changes, antimicrobial resistance, and climate change.3,4 Uruguay, located in southeast South America, has a temperate-humid climate with no dry season. It is part of the Pampas region, with grasslands as the predominant landscape,5 and is subject to droughts, floods, and extreme temperatures. Although it has a relatively high socio-economic and public health status and effective border health and preventive actions, it faces socio-economic challenges6 and health vulnerability to disease spread due to its penetrable borders and the increasing influence of climate change (increased temperature and rainfall) and El Niño/La Niña-induced climate variability,1,2,7 reflected in the emergence of diverse potential zoonosis sources with uncertain health repercussions.8
Here, we summarise the results for bat rabies, visceral Leishmaniasis, avian influenza, and Western Equine Encephalitis, which were recently reported.1 The main conclusions are summarised as follows:
Unfortunately, although mosquito-borne diseases such as Dengue and Chikungunya are on the rise in the country, there is a lack of available data in MGAP or WOAH reports; however, they are registered by the MSP and sent to PAHO. Though considering the growing concern at the national level since 2023, it would be advisable for these institutions to include these diseases in their reports and implement some form of coordinated monitoring and analysis under the One Health framework.
This research article focuses on the recently emerging and re-emerging Arboviral zoonotic diseases transmitted by Aedes aegypti, Dengue and Chikungunya. We analyse the outbreaks of the re-emerging and increasing ZIDS in Uruguay under the One Health framework based on three Research Questions (RQs):
RQ 1: Are the emerging zoonoses in Uruguay increasing, and why?
RQ2: Are the Arboviral diseases transmitted by Aedes aegypti becoming a Public Health concern or threat in the country?
RQ 3: How is the situation monitored, and could it be improved?
Finally, we compare the monitoring sources and reports and show their differences. Knowing the quality of reporting zoonotic infectious diseases and the necessary action is crucial in addressing this global health concern.1
We conducted descriptive statistical analyses of the Uruguayan reports of infectious diseases: WOAH, World Organisation of Animal Health (2016-2023), MGAP, Ministry of Agriculture, Livestock and Fisheries zoonosis reports (2016-2021), MSP, Ministry of Public Health notifications on zoonoses, and other relevant reports (e.g., Uruguayan Zoonosis Commission). Then, we summarised the most prominent emerging and re-emerging diseases and some of their climatic determinant factors analysed in our previous communication and conducted a contextual and historical review of the newly reported Arboviral zoonotic diseases transmitted by Aedes aegypti, Dengue, and Chikungunya based on the available data reported by the Uruguayan Ministry of Public Health (MSP) and the Pan-American Health Organization (PAHO). We compared the current reporting quality and consistency for the country's political-administrative divisions (19 Departments) (Figure 1).
Also, we deepened our investigation into their relationship to disease emergence by contrasting the WOAH and MGAP, highlighting differences in records (2016-2023), and improving the data and comments. Finally, we present preliminary data from the MSP Dengue and Chikungunya reports and discuss them.
Summary of the emerging and re-emerging infectious diseases in Uruguay
Table 1 summarises the emergence and re-emergence throughout 2004-2024 in Uruguay of four ZIDs, namely Bat rabies (since 2007), Visceral Leishmaniasis (2015), Highly Pathogenic Avian Influenza (2023), and Western Equine Encephalitis (2023), discussed by Vidal et al.1 It introduces those associated with Aedes aegypti, Dengue (since 2016) and Chikungunya (2023), which are the primary focus of this research article.
|
ZID and year of emergence or re-emergence |
Comment about the occurrence of ZIDs in Uruguay |
Sources |
|
Bat Rabies (2007) |
The country was declared rabies-free in 1960. However, an outbreak occurred between 1964 and 1968; the last human case was in 1966. In the 1980s, two canine rabies cases were reported. The introduction of infected vampire bats (Desmodus rotundus) from Brazil and changes in land use, particularly the habitat provided by the intensive cultivation of trees for cellulose production near the border, led to the first documented outbreak of paralytic rabies in livestock in the country. Only one bat species in Uruguay is hematophagous (Desmodus rotundus), and the colonies of rabies-infected insectivorous bats have recently increased, which threatens urban rabies reintroduction because Uruguay has not had rabies vaccination campaigns since 2001. Over 2022 and 2024, bats and a domestic cat—the first suspected species to jump from a bat rabies variant to a companion animal—were infected, and the lack of knowledge about the health status of stray animals and reservoirs precludes regaining the rabies-free status. |
|
|
Visceral Leishmaniasis (2015) |
Two sandfly species were first reported in the early 20th century. Still, no further specimens were found until 2010, when two male Lutzomyia longipalpis were discovered in Artigas and Salto. In 2015, a study in Salto found that 11 out of 49 dogs tested positive for the parasite. The first human case of visceral Leishmaniasis was confirmed in 2018 in a child from Salto. Subsequent studies showed that the disease mainly affected male dogs aged 2 to 4 years, with 86% exhibiting various clinical signs. Leishmaniasis cases were reported in almost half of the country, mainly in the north (Artigas and Salto). However, the endemic transmission was only confirmed in Salto, Artigas, and Rivera, showing the presence of vector and autochthonous cases. The maximum (2019) and minimum (2023) occurrence was associated with severe El Niño (high rainfall) and La Niña (low rainfall) climatic conditions; the latter was a historical extreme drought from 2021 to 2023, highlighting the potential role of climatic factors in the spread of Leishmaniasis. |
|
|
Avian Influenza (2023) |
Avian Influenza primarily affects birds, poultry, over 100 wild species, mammals, and humans. The first avian influenza cases were reported in Uruguay in February 2023, with dead black-necked swans (Cygnus melancoryphus) found on a coastal lagoon (Laguna Garzón) in Maldonado (likely induced by extreme La Niña-induced drought conditions), affecting both wild and domestic animals (e.g., swans (Cygnus sp.), turkeys (Pavo sp.), geese (Anser sp.), herons (Ardea sp.), and sea lions (Otaria sp.), leading to restrictions on the movement and trade of wild birds and their products, the declaration of a sanitary emergency, and the detection of the virus in marine mammals. The disease primarily affected the southern coastal region, except in Tacuarembó, with only one reported outbreak in March; however, it had the highest number of cases and deaths. Peaks in disease spread were observed in March and September, and some outbreaks involved multiple species, as seen in March in Flores and September in Canelones, where sea lions were also affected. |
|
|
Western equine Encephalitis (WEE) (2023) |
WEE primarily affects horses and, occasionally, humans. It is transmitted by infected mosquitoes, which acquire the virus from birds, with horses as incidental hosts. The disease typically occurs during warm and humid seasons but can persist year-round. The Western variant is present in Uruguay. Passerine birds serve as the primary reservoirs. Infected horses pose little risk of direct transmission to other organisms, making mosquito control essential for disease prevention. On December 2, 2023, the first WEE-reported case marked the onset of a significant sanitary event contained by May 2024. Twenty-five per cent of the infected horses died. The national equine population's morbidity rate was 0.26%, and the mortality rate was 0.09%. The disease spread rapidly and intensely between December 2023 and January 2024, and the most affected areas were low-lying, humid terrains with dense vegetation, bird presence, and abundant mosquito populations. By January 30, Uruguay reported its first human virus case in a decade in San José, a department with many cases in horses. The disease was detected in two-thirds of the country, with disease-free zones in the southwest and the southeast. The range of susceptible animals varied significantly, indicating high risk in certain areas. The incidence was notably concentrated in Río Negro, San José, Paysandú, and Artigas, bordering Argentina. |
|
|
Dengue, Chikungunya and Zika |
Uruguay had been free of autochthonous dengue cases since 1916 until an outbreak in 2016 saw 19 cases in Montevideo. Sporadic dengue outbreaks occurred in the following years. In 2023, the country experienced its first Chikungunya outbreak, with 60 cases in Paysandú after a period of high Aedes aegypti density. In 2024, Uruguay faced a significant dengue re-emergence, with 711 cases reported between January and May. Like previous ones, this outbreak followed a period of high mosquito density and peaked at the end of summer and early autumn. The growing number of Dengue and Chikungunya cases underscores Aedes aegypti's ongoing threat in Uruguay. No autochthonous Zika cases have been reported so far, though the country receives sporadically imported cases. |
Table 1 Summary of the emergence and re-emergence of Zoonotic Infectious Diseases (ZIDs), Bat rabies, Leishmaniasis, avian Influenza and western equine Encephalitis in Uruguay, 2004-2023. It is modified from Vidal et al.1 and introduces the ZIDs associated with Aedes aegypti, Dengue (since 2016), and Chikungunya (2023), which are the primary focus of this research article.
Monitoring and reporting of infectious diseases
Table 2 compares the monitoring sources and shows their differences. This table is a revised version of the one published before.1 We have found a few things that need to be corrected, as well as more inconsistencies among the reports that the responsible institutions need to improve.
|
Selected examples of differences between WOAH – MGAP and other reports |
||||
|
Year |
MGAP |
WOAH |
Others |
Notes |
|
2016 |
N/R |
1 OB - B. ab. - Rivera. January. |
- |
Possible occurrence (1-3 January). Public information is not available. |
|
1 OB – B. an. – Flores, March |
N/R |
- |
.- |
|
|
1 OB – B. ab. – Treinta y Tres, April |
N/R |
- |
- |
|
|
N/R |
1 OB – B. ab. – Salto, April |
- |
- |
|
|
N/R |
1 OB – B. an. – Paysandú, July |
- |
- |
|
|
N/R |
1 OB – B. ab. – Canelones, November |
- |
- |
|
|
1 OB – B. ab. – Paysandú, December |
N/R |
- |
- |
|
|
2017 |
1 OB – Tub. – Canelones, April |
N/R |
- |
- |
|
+10 OBs. B. ab. – (1 Artigas, 1 Cerro Largo, 3 Florida, 3 Paysandú, 1 San José, 2 Tacuarembó), June |
N/R |
- |
- |
|
|
1 OB – B. ab. – Rocha, December |
N/R |
- |
||
|
2018 |
N/R |
2 OBs. – Tub. – (1 Durazno, 1 Paysandú), January |
- |
- |
|
N/R |
1 OB – Tub. – Maldonado, February |
|
|
|
|
1 OB – Tub. – Durazno, February |
N/R |
|||
|
N/R |
2 OBs. – B. ab. – (Artigas), February |
|
|
|
|
N/R |
1 OB – Tub. – Maldonado, February |
|
|
|
|
2 OBs. – Tub. – (1 Maldonado, 1 Paysandú), March |
N/R |
|||
|
7 OBs. – B. ab. – (1 Maldonado, 2 Cerro Largo, 3 Artigas, 1 Paysandú), March |
N/R |
|||
|
1 OB – Tub. – Durazno, April |
N/R |
|||
|
N/R |
1 OB – Tub. – Durazno, May |
|
|
|
|
7 OBs. – B. ab. – (5 Tacuarembó, 1 Cerro Largo, 1 Artigas), May |
N/R |
|||
|
1 OB – Tub. – Paysandú, May |
N/R |
|||
|
N/R |
1 OB – B. ab. - Paysandú, June |
|
|
|
|
1 OB – Tub. – Flores, November |
N/R |
|||
|
1 OB – B. ab. – Rio Negro, December |
N/R |
|||
|
N/R |
1 OB – B. ab. – Rocha, December |
|
|
|
|
2019 |
- |
- |
- |
MGAP long period without reporting events (January 13 – February 9, 4 reports, 27 days) |
|
N/R |
1 OB – B. ab. – San José, March |
|
|
|
|
1 OB – Tub. – Paysandú, June |
N/R |
|||
|
MGAP period with intermittent event reports (weeks 22, 25, 27; 18 of 41 days free of zoonoses between late May and early July 2019) |
||||
|
6 OBs. – Tub. – (1 Durazno, 2 Flores, 3 Río Negro), July |
N/R |
|||
|
N/R |
1 OB – Tub. – Florida, July |
|
|
|
|
MGAP period with intermittent event reports (weeks 36, 37, 40, 41 and 43; 30 of 55 days free of zoonoses between early September to late October) |
||||
|
2 OBs. – B. ab. – (2 Río Negro), December |
N/R |
|||
|
2020 |
1 OB – B. an. – Río Negro, January |
N/R |
- |
- |
|
N/R |
1 OB – B. ab. – Cerro Largo, February |
|
|
|
|
N/R |
1 OB – B. ab. – Rocha, March |
|
|
|
|
N/R |
1 OB – B. ab. – Treinta y Tres, April |
|
|
|
|
1 OB – B. ab. – Rocha, June |
N/R |
|||
|
N/R |
1 OB – B. ab. – Paysandú, June |
|
|
|
|
1 OB – Tub. - Río Negro, June |
N/R |
|||
|
1 OB – B. ab. – Cerro Largo, August |
N/R |
|||
|
1 OB – B. ab. – Paysandú, October |
N/R |
|||
|
2 OBs. – B. ab. – (1 Tacuarembó, 1 Paysandú), November |
N/R |
|||
|
4 OBs. – B. ab. – (4 Canelones), December |
N/R |
|||
|
N/R |
2 OBs. – B. ab. – (1 Flores, 1 Paysandú), December |
|
|
|
|
2021 |
1 OB – B. ab. – Maldonado, January |
N/R |
||
|
1 OB – Tub. – Paysandú, January |
N/R |
|||
|
N/R |
1 OB – Tub. – Canelones, January |
|
|
|
|
2 OBs. – Tub. – (1 Treinta y Tres, 1 Colonia), February |
N/R |
|||
|
N/R |
1 OB – B. ab. – Treinta y Tres, February |
|
|
|
|
2022 |
- |
N/R |
1 OB – Rab. – 2 cases found in bats - Paysandú (since November)12 |
- |
|
2023 |
- |
N/R |
1 OB – Rab. - found in bat, Montevideo March 13 |
- |
Table 2 Monitoring sources differences (WOAH 2016-2023, MGAP 2016-2021). OB: outbreak / N/R: Not reported / B. ab.: Brucella abortus / B. an.: Bacillus anthracis / Rabies / Tub.: Tuberculosis
Source Modified from Vidal et al.1
Vidal et al.1 highlighted the timing problem of the WOAH and MGAP reports and their inconsistencies. Several minor mistakes impact the report’s accuracy and suggest a need for more control to correct human errors, which could be easily improved if reviewed. Besides, the WOAH reports, partly based on MGAP data, are released on a semi-annual time scale based—with monthly values—on weekly MGAP reports and need to be clarified to be fully understood. The WOAH should verify MGAP reports (and all the reports sent worldwide), which should update the missing information since March 2021 to follow up on the increasing trend in ZIDs.
Arboviral diseases transmitted by Aedes aegypti: Dengue and Chikungunya
After a continent-wide eradication program concluded in 1958, Uruguay remained free of Aedes aegypti until 1996, when a larva of this species was discovered in the city of Colonia del Sacramento (southwestern region, see #figFigure 1), signalling the reintroduction of the vector into the country.23 Since then, the infestation has spread across all departments, with a higher prevalence in those along the Uruguay River (bordering Argentina) and the border with Brazil.24 Currently, Aedes aegypti surveillance is conducted through a network of ovitraps in major cities, and the vector's activity is markedly seasonal (Figure 2).
Figure 1 Uruguay map showing the political-administrative division (19 departments)—source: Maps land (published under the Creative Commons Attribution-Share Alike 3.0 Licence).1
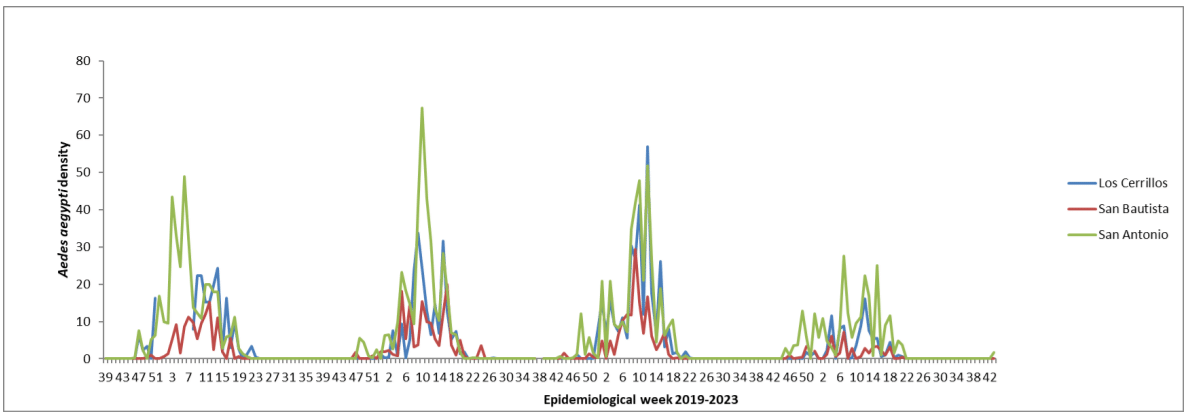
Figure 2 The average number of Aedes aegypti eggs per ovitrap in three cities of the Canelones department from 2019 to 2023.
Uruguay had been free from autochthonous dengue cases since 1916 when an epidemic occurred in the departments of Salto, Canelones, and Montevideo.25 However, in February 2016, an outbreak occurred in Montevideo, with 19 confirmed autochthonous cases.26 In the following years, small, sporadic dengue outbreaks were reported.25
In 2023, the country experienced its first outbreak of Chikungunya in the city of Paysandú between March and May, involving 60 cases following a high vector density period (Figure 3).
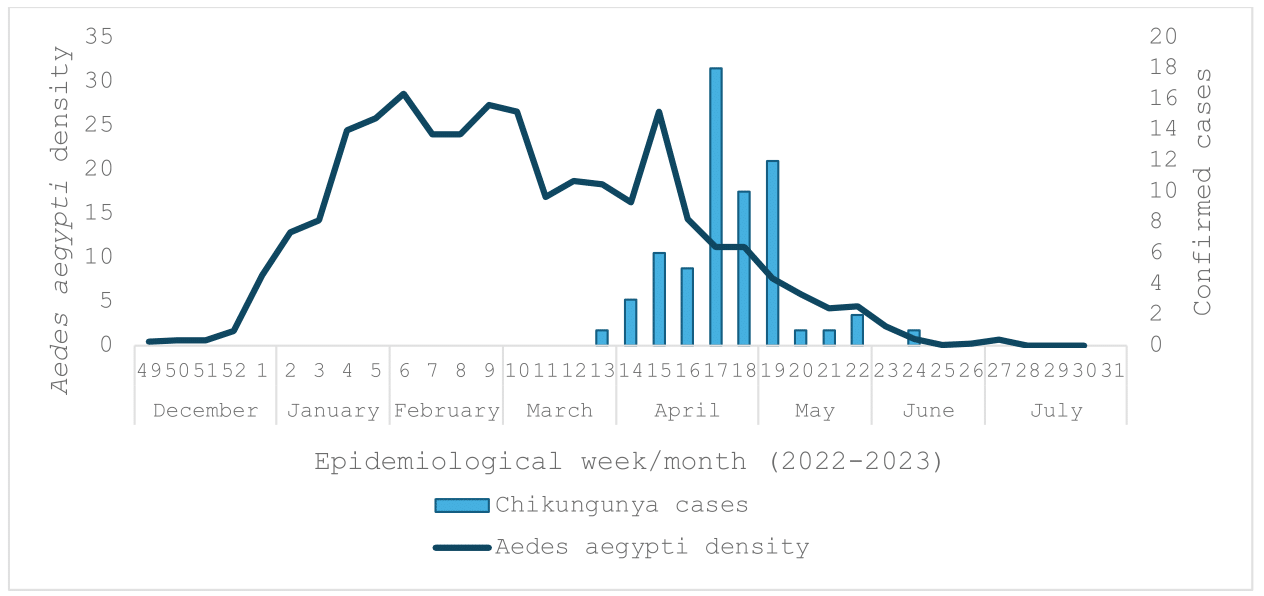
Figure 3 The average number of Aedes aegypti eggs per ovitrap and confirmed Chikungunya cases. Paysandú, 2022-2023.
In 2024, Uruguay faced a significant dengue re-emergence, with 711 cases reported nationwide between January and May. Like previous ones, this outbreak peaked after periods of high vector density, coinciding with the end of summer and the beginning of autumn (Figure 4).
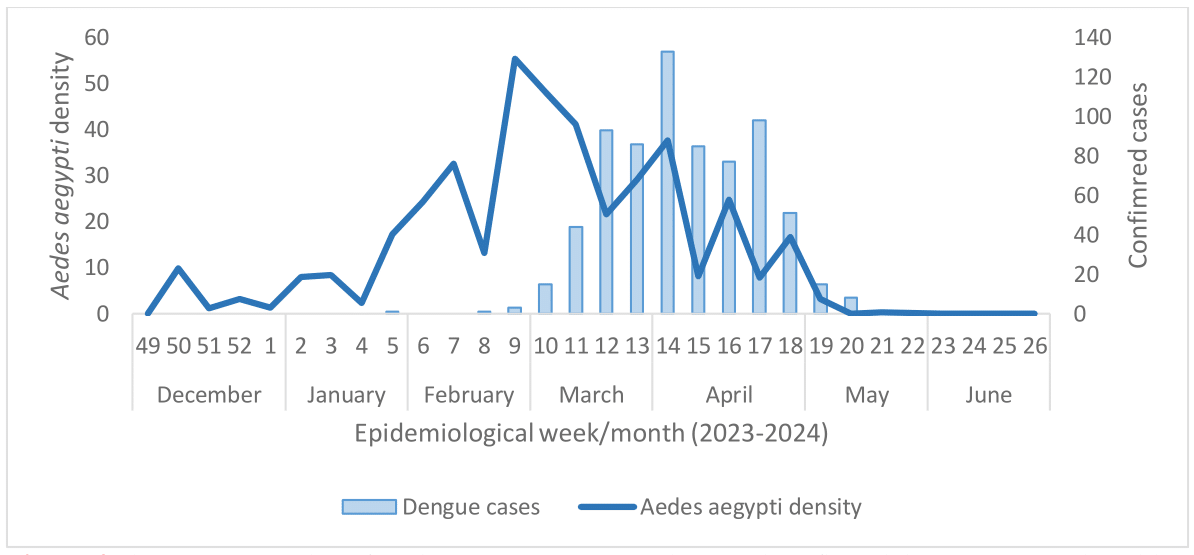
Figure 4 The average number of Aedes aegypti eggs per ovitrap and confirmed dengue cases nationwide, 2023-2024.
There have been no reported autochthonous cases of Zika so far; however, the country sporadically receives imported cases. High vector densities in late summer and autumn, combined with the history of disease transmission by Aedes aegypti, suggest a Zika outbreak is possible.
Control Strategies for the emergent and re-emergent ZIDs in Uruguay
Following Integrated Vector Management (IVM) notions, we reunited the main control measures that critical world and national institutions and local governments recommended. Integrated local measures should be planned efficiently, and base information is needed. Table 3 summarises basic control strategies for zoonotic infectious diseases (ZIDS).
|
ZIDs |
Control Strategies |
Sources |
|
Rabies |
1. Raising awareness of the problem to the whole society is desired and empowers the community. Information about vaccination, prophylaxis, life cycle, and epidemiology should be provided broadly. The One Health approach is required, and awareness about the interconnection between wildlife, animal, and human health is crucial for adequate community response. Wildlife should be respected, and potentially infected or symptomatic animals should be reported to the authorities. 2. Implement mass vaccination campaigns for dogs and cats. Strengthen bat population surveillance programs, including capturing and analysing samples for the presence of the virus. Include these data in Reports (several countries make a special effort in the bat population for disease control). 3. Develop awareness programs about vaccinating pets. Implement stray dog and cat population control programs to reduce the possibility of transmission (the wildlife population should also be considered). 4. Cross-border control policies: Improve border surveillance, especially with Brazil, to prevent the entry of infected bats. |
|
|
Leishmaniasis |
1. Vector Control: Implement fumigation and vector control programs in affected urban and rural areas. Sandflies emerge from eggs in habitats rich in organic waste (plant parts, nets, manure, grass). The peri-domiciliary area should be evaluated and controlled, and domestic houses must be improved (nets should be added, with orifices lesser than 4-5 mm2). Human protection is another effective control measure. 2. The control of infected dogs (stray and familiars; responsible tenure, veterinary care, bedding, collar repellents, net usage, control dog behaviour), surveillance with periodic veterinary check-ups and awareness campaigns regarding the disease and the sandfly should be intensified (e.g., use of mosquito nets and repellents and increase population awareness). 3. Create early warning systems based on climatic factors to prevent leishmaniasis outbreaks. |
|
|
Avian influenza |
1. Establish strict controls over poultry farms, including quarantines, vaccination programs, and continuous health monitoring (it affects poultry and 100 wildlife species, especially waterfowl). Impose restrictions on the marketing and movement of wild birds and poultry products. 2. Establish surveillance programs for migratory birds that may carry the virus (wildlife monitoring, including mammals). 3. Raise social awareness about direct or close contact with potentially infected birds or animals, being the best prevention to avoid sources of exposures (saliva, mucous, faeces, secretions and infected animals; infection can happen air-driven, in droplets or dust, and could enter through eyes, nose or mouth). Infection commonly happens when people maintain prolonged and unprotected contact with infected organisms (nor gloves or protection). People should always avoid direct contact with sick or dead wild birds or poultry, as well as surfaces or material contaminated and consistently denounce the situation to the authorities. |
|
|
Equine encephalitis |
1. Establish mass equine vaccination programs for horses in rural and affected areas. Implement mosquito control programs, especially and strategically in wetlands. Carry out surveillance programs in the mosquito and bird population to detect the presence of the virus. Include these registers with the WOAH. 2. Develop awareness programmes among the rural population and promote eliminating excess sources of stagnant water in rural areas. 3. Authorities should pay particular attention when environmental conditions (e.g., the rainy spring season and extensive flooding in 2023 in Uruguay), the characteristics of vertebrate hosts (e.g., avian migratory species), and vectors need further investigation. 4. Analyses about the genres of mosquitoes observed and with infective capacities should be analysed since more than 70 species of mosquitoes are described in the country. After identifying them, specific species, particular behaviours, or environmental relations should be identified, and actions should be taken based on the specific vectorial control. |
|
|
Dengue, Chikungunya and Zika |
1. Control of the vector, Aedes aegypti, is one of the fundamental pillars of disease control, and protection from mosquitoes and their bites is essential: avoid viable life cycle (using chemical, biological or environmental controls, for adults or larvae), surveillance (monitor populations, maintain communication and multisectoral efforts), raise awareness about the genre (activity during the day, outdoor and indoor hazard), encourage repellent use, adequate clothes (loose-fitting and long-sleeved shirts and pants, primarily light coloured), nets (ideally sprayed with repellent) and screens. Public education is crucial (involving the community within management, as the Integrated Vector Management IVM). Enhance arbovirus surveillance, as an example of a specific effort (identifying high-risk transmission zones, mapping, characterising areas with high mosquito density and human susceptibility), is necessary, and efforts should be incredibly intense during high-risk periods (late summer and autumn). Previous education and training are needed. 2. If travelling to a dengue/zika/chikungunya-positive territory, pay special attention and awareness; read all the sanitary protocols and take all the migratory considerations. Interiorising symptoms to identify a risk situation quickly before causing sanitary damage and seeking medical advice as soon as possible. Risk populations such as pregnant women and children should take extra measures. 3. In outbreaks, vector control and efforts to stop transmission in the area should intensify. PAHO, in response to cases related to Aedes aegypti, urges Member States to provide adequate healthcare services, reorganise institutions, strengthen networks, and improve laboratory diagnosis capacities, training and case management. Insecticide resistance should also be evaluated, and rational use of insecticides should be ensured while promoting the development of sustainable controls such as the Sterile Insect Technique (SIT) or the Incompatible Insect Technique (IIT). |
Table 3 Control strategies for the emergent and re-emergent ZIDs in Uruguay.
Source The authors, based on the cited references.
We detected the need for improved and consistent monitoring and reporting and the One Health approach in public health. The re-emergence of diseases such as rabies in bats and transmission to domestic animals underscores the need for constant vigilance and appropriate vaccination campaigns. This should always be associated with cross-border international collaboration, including international organisations and adopting effective protocols (such as improved reporting), which must also consider wild and stray animals (a recognised problem in Uruguay). Disease monitoring and reporting in veterinary clinics could be an exciting option. The lack of vaccination campaigns at various levels is evident, increasing the risk of outbreaks.
Due to the inconsistencies shown in the current reports, it could be helpful to develop a One Health vision reporting (including data on zoonoses in animals and humans, including all WOAH data, Dengue, Chikungunya and other diseases that would require a new way of collecting data, added to other minor ones linked to small animals). Wildlife health should also be deepened, with randomised studies of wild animals included in these reports (e.g., rabies in bats, reproductive and respiratory syndrome in wild boars, and rabies in canids).
Within the One Health framework (compared to the United States, which has programs such as the National Wildlife Health Centre, for example), the control of zoonoses in wildlife in Uruguay needs to be improved. More financial and technological resources are needed to improve zoonosis monitoring systems and research. Notions such as IVM (Integrated Vector Management) should be interiorised, and involved actors (e.g. MSP, MGAP, SINAE) should synergise more efficiently, maybe through a new One Health agency or institution.
The general population's lack of knowledge about zoonoses reinforces the need for public awareness campaigns. One health should be added to the academic curriculum of secondary and tertiary institutions.
Additionally, rodent-borne Leptospirosis - an endemic zoonosis in Uruguay -shows correlations between the annual number of cases and accumulated annual precipitation,7 highlighting the potential to address the impact of ZIDs by understanding the role of climate as a primary determinant factor in the country.
Regarding the RQ 1: Are the emerging zoonoses in Uruguay increasing, and why?
This research article and the previous short communication underscore the urgent need to address the increasing trend of emerging and re-emerging ZIDs, highlighting the potential impact of our research. Also, the reported occurrence of Leishmaniasis and Avian Influenza —as well as Leptospirosis— associated with the precipitation suggests that understanding the role of climate is crucial in addressing the impact of ZIDs. Therefore, our team is analysing how temperature and rainfall influenced Leptospirosis occurrence for 2010-2023, further emphasising the pressing need for action.
Regarding RQ 2: Are the Arboviral diseases transmitted by Aedes aegypti becoming a Public Health concern or threat in the country?
The available data since 2023 indicates a worrying trend in the density of Aedes aegypti and the occurrence of autochthonous cases of Dengue and Chikungunya, along with their imported ones (including Zika), are on the rise, suggesting a looming threat of Zika outbreaks, underscoring the gravity of the situation.
Regarding the RQ 3: How is the situation monitored, and could it be improved?
While ZIDs are being monitored and reported, our research has identified several areas for improvement. These include addressing mistakes, inconsistencies, and delays and enhancing inter-institutional coordination between MGAP, WOAH, MSP, and PAHO. By implementing these improvements, we can ensure more accurate and timely reporting of ZIDs, enhancing our ability to respond effectively to these diseases.
None.
The authors declare there is no conflict of interest.

©2024 Vidal, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.