MOJ
eISSN: 2379-6383


Research Article Volume 9 Issue 1
1Halberg Hospital and Research Institute, India
2PJ Safaric University, Slovakia
3Comenius University, Slovakia
4Everglade University, USA
5Department of Community Nutrition, Faculty of Human Ecology, IPB University, Indonesia
6Life Science Institute, Japan
7Department of Food Science and Technology, Banaras Hindu University, India
8Department of Biotechnology, ERA Medical College, India
Correspondence: Ram B Singh, Halberg Hospital and Research Institute, Moradabad, India, Tel 919997794102
Received: December 12, 2019 | Published: February 12, 2020
Citation: Singh RB, Fedacko J, Mojto V, et al. Effects of millet based functional foods rich diet on coronary risk factors among subjects with diabetes mellitus: a single arm real world observation from hospital registry. MOJ Public Health . 2020;9(1):18-25. DOI: 10.15406/mojph.2020.09.00318
Introduction: Diabetes mellitus has become a global public health problem, characterized by increased intake of western style diets and decline in physical activities which are pro-inflammatory. Food diversity, nutrient profile, glycemic index and lower content of salt sugar and Tran’s fat are an important consideration for a healthy anti-inflammatory diet which may be advised for prevention of diabetes mellitus and cardiovascular diseases (CVDs). This clinical observation aims to examine the effects of a millets based functional food rich intervention diet on coronary risk factors among subjects with known diabetes.
Method: After permission from the review board of a hospital, hospital records of 65 subjects with type 2 diabetes mellitus were drawn for this study. Of 65 patients with diabetes, 5 were excluded and remaining 60 were administered millet-based functional food rich intervention diet (millets 60%, soya bean 20%, brown rice 10%, peanuts 8% and flex seeds 2%). Clinical data, dietary intakes and physical activity were assessed by validated questionnaires. Blood pressures were measured by sphygmomanometer.
Result: Treatment with millet based intervention diet for 12 weeks was associated with a significant decline in fasting and 2-hour postprandial blood glucose, HbA1c indicating that this diet can prevent diabetes. Total cholesterol, VLDL cholesterol and triglycerides showed a significant decline compared to baseline levels. Pro-inflammatory cytokines; C-reactive proteins, TNF-alpha and interleukin-6 also showed significant reduction after treatment with intervention diet compared to baseline levels. In association with these changes, there was a significant decline in systolic and diastolic blood pressures, parameters of oxidative stress; TBARS, MDA and diene conjugates with an increase in antioxidant vitamins; A,E and C and beta-carotene. Underlying these changes, all subjects received an 11 fold greater amount of millet-based intervention diet which increased from mean 21.36±3.8g/day to 235.20±23.6 (p<0001).Among females (n=33), there was a significant increase in hemoglobin and serum calcium and magnesium indicating that millet based diet can also prevent under nutrition.
Conclusion: It is possible that millet-based intervention diet can cause a significant decline in blood glucose, HbA1c, oxidative stress, blood pressures, blood lipoproteins and pro-inflammatory cytokines with an increase in antioxidant vitamins, magnesium, calcium and hemoglobin. Randomized, controlled intervention trials, would be necessary to confirm our findings.
Keywords: hyperglycemia, dyslipidemia, cytokines, nutrition, inflammation, whole grains
The increase in morbidity and mortality due to cardiovascular diseases (CVDs) and diabetes as well as due to other chronic diseases, maybe due to unhealthy Western-style diets and decline in physical activity which are known to predispose chronic low-grade inflammation.1–7 Approximately 50% of mortality due to non-communicable diseases(NCD) has been attributed to an unhealthy diet in all the six continents of the world by the UNO and WHO.1–7 These changes in diet and lifestyle are associated with obesity, central obesity and metabolic syndrome(MS) which predispose increased risk of NCDs. The possible mechanisms are oxidative stress, deficiency in antioxidant status, hyperglycemia, dyslipidemia and increase in inflammation in the endothelial cells, beta cells of pancreas, neurons and hepatocytes leading to CVDs and other chronic diseases.4–7 Cohort studies and randomized trials confirm that Indo-Mediterranean foods such as vegetables, fruits, whole grains, seeds and nuts in conjunction with olive oil, rapeseed oil or a blend of oils may be protective against NCDs.6–12 It seems that food diversity along with a nutrient profile of foods is the basic principle of functional food security (Figure 1).
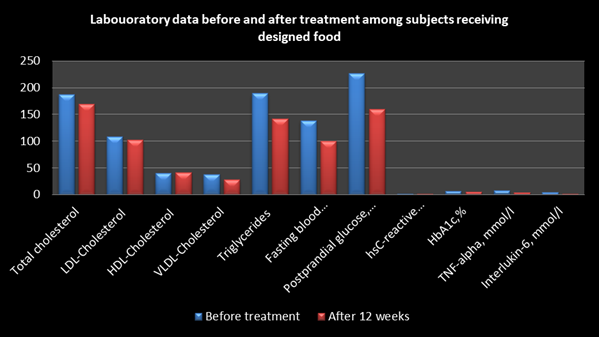
Figure 1 Effects of millets based diet on blood glucose, blood lipids and pro-inflammatory cytokines.
Cohort studies have also demonstrated that control of under nutrition and hunger by increased supplementation with western type foods has been associated with a decline in under nutrition but with the rapid emergence of NCDs.1–3,6,7 In most developing countries, including India, under nutrition is still common and one-third of the children and women have iron, calcium and protein deficiency.1–3,13–19 There is a need to develop functional designer diets, based on inexpensive whole grains, such as millets which could be protective to health and friendly to the environment.10–13 Recently, millets, brown rice or medical rice, soybeans, flax seeds, peanuts, as well as rice bran oil and rapeseed oil have been found to protect against diseases and for health promotion.13–20 It is hypothesized that a combination of these foods in the form of a designer food could be used for intervention against hunger in developing countries and against NCDs in middle and high-income countries due to nutrients profile of millets. Food diversity and nutrient profile are the most important considerations when we design a functional designer food. Other aspects are glycemic index, the content of salt, sugar, trans fat and peroxidised fat in the final product which can be taken care of in this food. This real-world clinical observation aims to report the effects of millets based functional foods intervention on coronary risk factors among patients with diabetes, to emphasize its role in the prevention of CVDs and diabetes.
After permission from the review board of Halberg Hospital and Research Institute, Moradabad, India, records of 65 subjects with type 2 diabetes mellitus, receiving millet-based designer food diet were drawn for this study. Informed consent from all the subjects was taken before advising diet. All subjects were registered after permission from Halberg Hospital and Research Institute Ethics committee, Moradabad, India. Clinical observations were recorded during the last one year from April 2018 to April 2019. Only known subjects with type 2 diabetes mellitus, with a previous record of diagnosis and/or drug therapy; metformin and/or other drugs, were included in this observation. Of 65 subjects volunteered, 5 were excluded and 60 participants (33 females and 27 males) received millet-based designer’s foods for the trial.
Exclusion criteria
Exclusion criteria were cancer, chronic dysentery, HIV, metabolic diseases unrelated to diabetes, unable to give consent or did not satisfy the criteria of diagnosis of the trial.
Inclusion criteria
Adults above 30 years without recent AMI or stroke, with any of the markers of type 2 diabetes mellitus (records of taking hypoglycemic drugs) or fasting blood glucose >140mg/dl or postprandial blood glucose 200mg/dl and above or randome blood glucose >126mg/dl.
Intervention
In India, it’s easy to provide desired intervention diet because majority of the people traditionally consume an optimal amount of total fat and saturated fat similar to advise of the National Cholesterol Education Program Step 1 diet.9,11 Since whole grain consumption in Indians is lower (200-250g/day) than advised by the WHO (>400g/day), we administered 200-250 g/day of designer foods (millets 60%, soya bean 20%, brown rice 10%, peanuts, 8%, flax seeds, 2%) in this observation of 12 weeks. These foods are rich sources of dietary fiber, essential and non-essential amino acids, poly phenolics and flavonoids.14–20 Millets are also rich in calcium, magnesium, zinc, copper and iron. Soya beans are also rich in isoflavons and omega 3 fatty acids. Brown rice is rich in flavonoids and resistant starch. Flex seeds are rich in omega3 fatty acids and peanuts in monounsaturated fatty acids. The glycemic index of these foods is lower than 40%. The flour of these foods was consumed in the form of Indian bread, roasted bread in oil (paratha), roasted vegetables covered with designer flour (pakora) and cakes and low salty snacks. These dishes are known to have a low glycemic index. Low salt and low trans fat. Low added sugar was advised. with substitutes of honey, raisins and dates in sweetened food products like cakes and halwa (flour is roasted in slow heat along with oils or clarified butter and finally the water is added to make a paste along with sweetening foods like honey, raisins and dates) The lack of palatability of preparations was taken care of by allowing the subjects to use minimal amount of tomato or green sauce (10-12g/day), sweet fruits raisins, dates, and honey rather than added sugar (10-20g/day).
Clinical data such as age, sex, height, weight, waist circumference, blood pressure and behavioural risk factors, dietary intakes, physical activity, tobacco intake and alcoholism were recorded in all the subjects via a case record form during the assessment period of one week, in which all subjects were advised to continue the same diet.
Criteria for diagnosis of risk factors
Pre hypertension was considered in the presence of systolic blood pressure, 130-139 mm Hg and diastolic 85-89 mm Hg. Pre-diabetes was considered in the presence of fasting blood glucose 110-125 mg/dl. Central obesity was considered in the presence of waist circumference of 90 mm and above in men and 80mm and above among women. Presence of any three or more criteria was considered as metabolic syndrome.
Collection of data such as body weight, waist circumference, blood pressures, hypoglycemic episodes, etc. were recorded in all the subjects during the follow-up. Blood pressures were recorded by mercury manometer in the sitting position after 5 min rest. Blood Pressures were measured three times and an average of two last readings was taken as final blood pressure. Data on food intake, tobacco and alcohol intake and physical activity were obtained by validated questionnaires. Food intakes were obtained by dietary diaries completed by each subject for 7 days and assessed by a dietitian using food models, food portions and food measures.
Follow up
The follow-up period for all the patients was for 12 weeks. Food intakes were closely monitored and standardized by advising the subjects to eat the same diet except for reducing refined foods and sweetened food products and red meat. The aim was to ensure that refined foods and sweetened food products and red meat are replaced by the intervention of food products which were given in the form of traditional diets. The dietary advice was reinforced by showing food models, food measures and food portions to keep food and energy intakes as close to the target as possible. Non-adherence to the feeding protocol was assessed and reinforced by phone calls or weekly visits by the dietitian. Those subjects who were taking a lesser amount of advised foods were suggested to supplement the foods with other tasty spices and tomato sauce (salted foods) and sweet fruits like bananas, papaya, raisins and dates in case of sweet diets.
Physical activity was assessed by validated questionnaire and included both occupational and spare time activity scores- Subjects were classified being sedentary or performing a mild, moderate and or heavy activity.
The laboratory data were obtained following an overnight fast and taken between 8-9 AM. TBARS, MDA, diene conjugate, vitamins E and C, nitrate, and angiotensin-converting enzyme were measured by colorimetric methods using UV-VIS Spectrophotometer, 320 to 11 nm (Electronics Corporation of India, Ltd). Routine blood tests such as serum creatinine, serum bilirubin, blood counts and, Hb, ESR were done in all subjects. Glycosylated hemoglobin (HbA1c) was assayed with Bio-Rad kit (Bio-Rad Laboratories, Inc, Hercules, CA). Lipid profiles were determined by Pictus 500 (Medicine Hellas; Location). TNF-α and IL-6 were analyzed on Vidas machines (Vidas Biomerieux, Marcy I'Étoile, France).
Statistical analysis
Data were analyzed by paired t-test in case of ordinal variables or Students t-test for continuous variable. Only P values<0.05 and two-tailed t-test were considered significant.
All 60 subjects (33 females and 27 males), included in this analysis were followed up for 12 weeks. Of total 60 subjects, approximately half (n=31) mentioned about the lack palatability of preparations made out of this whole-grain flour food products, resulting into lower intake(10-30% of these foods which was prevented by allowing the subjects to take tomato sauce (10-12g/day, low salt foods), sweet fruits raisins, dates, honey (10-20g/day, for sweet preparations). Table 1 shows that the consumption of whole grains was lower than the advice of WHO (400g/day) which was increased from mean 231.5±15.82 g/day to 422.5±25.82 g/day by increased supplementation of designers intervention foods (235.20±23.6 g/day). Supplementation of these foods was associated with a significant decline in the intake of refined foods (from 216.833±12.93 to 15.33±3.29 g/day) and sweetened food products from 47.36±5.045g/day to 23.36±3.04g/day. The consumption of total fatty acids, and total visible fat, fruits and vegetables and roots showed no significant differences. Body weight, body mass index, waist and waist hip ratio revealed a slight non-significant decline in these parameters after supplementation of intervention foods for 12 weeks. However, systolic and diastolic blood pressures and heart rate were significantly reduced at 12 weeks compared to levels at baseline (Table 2).
Food and nutrient intake |
Baseline |
After 12 weeks |
P value |
Total Fatty acid, g/day |
220±11.05 |
218±11.55 |
0.145 |
Total Visible fat, g/day |
24.66±2.64 |
23.76±2.54 |
0.244 |
Total trans fat, g/day |
5.1±0.63 |
3.6±0.33 |
0.105 |
Meats & egg, g/day |
15.1±2.23 |
12.1±3.23 |
0.052 |
Fruits & vegetables, g/day |
211.7±10.83 |
215.7±14.83 |
0.721 |
Whole Grains, g/day |
231.5±15.82 |
422.5±25.82** |
0.001 |
Millets, g/day |
4.32±0.64 |
124.36±20.14** |
0.001 |
Soy beans, g/day |
5.36±1.04 |
47.36±6.04** |
0.001 |
Flex Seeds. g/day |
0.36±.04 |
8.36±0.04** |
0.001 |
Brown rice, g/day |
4.16±1.04 |
17.76±3.04** |
0.001 |
Nuts, g/day |
7.16±1.34 |
37.36±6.045** |
0.001 |
Total of designer foods, g/day |
21.36±3.8 |
235.20±23.6** |
0.001 |
Roots, g/day |
247±9.85 |
235±9.26 |
0.062 |
Refined foods, g/day |
216.833±12.93 |
15.33±3.29* |
0.001 |
Milk & curd, g/day |
114.66±8.78 |
112.166±6.73 |
0.321 |
Sweets, g/day |
47.36±5.045 |
23.36±3.04* |
0.015 |
Salt, g/day |
7.36±5.045 |
6.45±5.045 |
0.143 |
Total carbohydrates,% K calories/day |
55.3±6.2 |
61.5±7.4 |
0.088 |
Total proteins, % K Calories /day |
12.1±2.4 |
13.7±2.5 |
0.096 |
Total fat, % K calories/day |
27.5±3.5 |
29.4±4.0 |
0.085 |
Total energy, K calories/day |
2078.76±54.32 |
2066.67±56.27 |
0.467 |
Physical activity scores |
3.87±0.29 |
3.86±0.2649 |
0.241 |
Table 1 Food and nutrient intakes and physical activity scores, before and after treatment with designed diet
*=P<0.05, **=P<0.01, values are obtained by comparison of baseline verses after intervention, 12 weeks
Variables |
Before treatment |
After Treatment |
p-value |
Age |
44.4±9.160 |
44.4±9.16 |
0.9999 NS |
Male sex, n(%) |
24(40.0) |
24(40.0) |
|
Height (Cm) |
155.966±8.799 |
155.966±8.79 |
0.9999 NS |
Weight(Kg) |
71.54±12.207 |
68.126±11.74 |
0.4822 NS |
Body Mass Index, Kg/M2 |
28.921±4.809 |
27.167±11.84 |
0.05477 NS |
Waist,cm |
98.733±10.635 |
95.2±9.13 |
0.1708 NS |
Hip,cm |
99.3±10.176 |
96.7±9.37 |
0.3075 NS |
Waste/hip ratio |
0.9963±0.07663 |
0.988±0.06 |
0.6610 NS |
Heart rate, per min |
86.7±7.284 |
82.126±5.13 |
0.050* |
Blood Pressure, mm Hg |
|
|
|
Systolic |
127.33±10.66 |
122.25±5.07** |
0.01 |
Table 2 Clinical characteristics of subjects before and after receiving designed foods
NS=Non-significant, *= < 0.05, Significant, **, P <0.01
Mean concentration of total cholesterol, VLDL cholesterol were optimal with high triglycerides at baseline, which were significantly declined after intervention for 12 weeks (Table 3). Fasting and post prandial blood glucose and HbA1c also revealed significant decline after intervention for 12 weeks compared to baseline levels. C-reactive proteins, tumor necrosis factor (TNF)-alpha and interleukin-6 that are pro-inflammatory cytokines also showed significant reduction after intervention diet compared to baseline levels.
Laboratory data |
Before treatment |
After 12 weeks |
P value |
Total cholesterol, mg/dl |
187.67±32.420 |
169.226±26.056 |
0.0183S |
LDL-Cholesterol |
109.0233±34.447 |
102.4733±28.005 |
0.4223NS |
HDL-Cholesterol |
39.726±4.203 |
41.379±1.776 |
0.1113NS |
VLDL-Cholesterol |
37.336±17.881 |
27.993±11.313 |
0.0187S |
Triglycerides |
189.8±93.257 |
142.396±65.033 |
0.0261S |
Fasting blood glucose, mg/dl |
138.503±40.97 |
100.066±23.13 |
0.001S |
Postprandial glucose, mg/dl |
226.54±47.2033 |
160.49±30.14 |
0.0028S |
hsC-reactive proteins, mmol (0-1.0) |
1.548±0.2033 |
1.476±0.1487 |
0.262S |
HbA1c, % |
6.866±0.8891 |
5.073±0.1799 |
0.0001S |
TNF-alpha, mmol/l (0-8.1) |
7.810±1.221 |
3.9±0.649 |
0.0007S |
Interlukin-6, mmol/l(0-5.0) |
4.933±0.7024 |
1.606±0.541 |
0.01667S |
Table 3 Laboratory data before and after treatment among subjects receiving designed foods
S=Significant, NS=Non-significant
Table 4 shows the concentrations of antioxidant vitamins and parameters of oxidative stress before and after 12 weeks of intervention. The levels of TBARS, MDA, and diene conjugates indicating oxidative stress were significantly declined, whereas antioxidant vitamins; A, E, and C, as well as beta- carotene, revealed significant increase after treatment compared to baseline levels.
Data |
Before treatment |
After 12 weeks |
P-value |
TBARS, mmol/l |
2.454±0.2822 |
1.596±0.3250 |
0.0001 S |
MDA, mmol/l |
3.396±0.1968 |
2.605±0.1848 |
<0.0001 S |
Diene. Cojugates, OD |
27.756±0.9947 |
24.663±0.4767 |
<0.0001 S |
Vitamin E, mmol/l |
23.033±2.312 |
28.766±1.906 |
<0.0001 S |
Vitamin C, mmol/l |
20.7±2.466 |
24.2±1.846 |
<0.0001 S |
Nitrite, µ mol/l |
0.628±0.058 |
0.676±0.06100 |
0.0034 S |
ACE, µ mol/l |
85.233±7.176 |
75.233±7.319 |
0.0293 NS |
Table 4 Antioxidant vitamins and oxidative stress among subjects receiving designed foods
Values are S=Significant, NS=non-significant
Of 60 subjects with diabetes mellitus, 33 were females whose baseline levels of hemoglobin and serum calcium were lower than normal values (Table 5). There was a significant rise in hemoglobin, iron and magnesium, after 12 week of intervention with intervention foods compared to baselines levels. No significant changes were observed in these parameters, when data were analysed only among, men.
Data |
Before treatment (n=33) |
After 12 weeks (n=33) |
P value |
Hemoglobin g/dl |
10.554±04.28 |
13.59±4.42 |
0.040S |
Serum proteins, g/dl |
4.39±1.19 |
5.605±1.58 |
<0.060NS |
Serum calcium, mg/dl |
8.75±3.89 |
9.2±3.07 |
<0.050S |
Serum magnesium, mg/dl |
1.53±0.33 |
1.76±0.45 |
<0.040S |
Serum iron, mmol/l |
15.23±3.17 |
22.33±4.32 |
<0.0293S |
Table 5 Biochemical data among women subjects receiving designed foods
S=Significant, NS=Non-significant
In view of the beneficial effects of certain inexpensive foods such as millets, there is a need to study the impact of functional food security via increased production and consumption of millets with reference to sustainability and health, among populations, in both developed and developing countries. Since food diversity, that addresses nutrient profile and food profile, is the basic principle of functional food rich healthy diet, it is considered an intervention diet for this clinical trial.9–15 This study shows that millet based functional food rich intervention diet, may cause significant decline in blood glucose and HbA1c that are important markers of diabetes (Table 3). There was a significant decline in total cholesterol, VLDL cholesterol and triglycerides along with pro-inflammatory cytokines, C-reactive proteins, TNF-alpha and IL-6. These findings indicate that intervention with designer’s foods, may inhibit glucose production and increase glucose utilization, due to possible improvement in insulin sensitivity by these foods.8–15 The decline in blood lipoproteins and pro-inflammatory cytokines indicate that this diet also has anti-inflammatory effects which may be due to increased consumption of poly phenolics and flavonoids as well as antioxidant vitamins and dietary fiber and a lower intake of refined foods and sweetened food products (Table 1&4).
Millets are rich sources of dietary fiber and flavonoids and polyphenolics which have been demonstrated to provide benefits in diabetes and CVDs.14-20 A recent study, investigated on the natural antioxidants in edible flours of small millets. Total carotenoids content varied from 78–366μg/100 g in the millet varieties with an average of 199±77, 78±19, 173±25, and 366±104 μg/100g in hemoglobin finger, little, foxtail, and proso millets respectively.17 HPLC analysis of vitamin E indicated a higher proportion of γ−and α-tocopherols; however, it showed lower levels of tocotrienols in the millets.14 Total tocopherol content in finger and proso millet varieties were higher(3.6–4.0 mg/100 g) than in foxtail and little millet varieties(∼1.3mg/100g). Total antioxidant capacity in finger, little, foxtail and proso millets were 15.3±3.5, 4.7±1.8, 5.0±0.09, and 5.1±1.0mm TE/g, respectively. It is possible that edible flours of small millets are a good source of endogenous antioxidants.17
There are only a few studies, to demonstrate the beneficial effects of millets on CVDs and diabetes in human beings. However, experimental studies indicate that millets can exert antioxidant effects and reduce oxidative stress and hyperglycemia.21–25 These beneficial effects of millets may be due to their composition and enzyme inhibitory properties of seed coat phenolics which may cause inhibition of Α-Glucosidase and pancreatic amylase indicating protection of beta cells of the pancreas.23 Another experiment, with finger millet feeding, during the process of wound healing, revealed beneficial effects in diabetic rats, by decreasing blood glucose and healing process.24 In hyperlipidemic rats, millet consumption was associated with a significant reduction in serum concentration of triglyceride and C-reactive protein without any effects on oxidative status.25 The decline in triglycerides and C-reactive protein is similar to our study, whereas no effect on oxidative stress is surprising because millets are rich in antioxidant polyphenolic, flavonoids and carotenoids.14–24
The intervention subjects showed non-significant decline in BMI, (from 28.921±4.809 to 27.167±11.84), hence a part of the benefit in biomarkers could possibly be due to weight loss. A substantial change in SD suggests that a few subjects may possibly have significant decrease in body weight. These effects on blood pressures, HbA1c, lipids, oxidative stress and inflammatory factors are known to relate with obesity. It is possible that a part of the benefit on BMI and biomarkers may be due to reduction in BMI, rather than the effects of functional foods. A larger study with longer follow up or a randomized, controlled clinical trial would be necessary to confirm this presumption.
In a population survey on diet and nutritional status of rural population and prevalence of hypertension among adults in rural areas revealed, that increased intake of millets was associated with decreased prevalence of hypertension.26 No other study has examined the antihypertensive effects of millets in human beings. Our study also showed a significant decline in both systolic and diastolic blood pressure after 12-weeks administration of millet-based functional food intervention (Table 2). The decline in blood pressures may be due to a decline in angiotensin-converting enzyme and an increase in nitrate (Table 4). While angiotensin-converting enzyme is known to cause smooth muscle cell dysfunction, nitrite acts by increasing NO (nitric oxide) release which is a vasodilator. Millets can also protect genetic and epigenetic damage and enhance microbiome which may be the additional mechanism for beneficial effects of such diet, against CVDs and diabetes as well as cancers.27,28 Finally, our study, also found that this millet based intervention diet caused a significant increase in serum iron and haemoglobin and calcium indicating that it can be beneficial in combating under nutrition in females and possibly osteoporosis in both genders (Table 5).29,30
Apart of above, beneficial effects of the intervention diet may also be due to increased intake of isoflavones and other antioxidant flavonoids found in soybeans, brown rice, peat nuts and flax seeds which are rich in omega-3 fatty acids.31–34 Soybeans are rich sources of nonsteroidal phytoestrogenic and antioxidative diphenolic compounds with potential roles in the prevention of chronic diseases, including hormone-dependent cancer, CVDs, osteoporosis and postmenopausal syndrome.31, 35–37 Japanese consume 40-100 mg/day from various kind of soy foods, and it may contribute to the low incidence of the above diseases. S(-)-equol, a metabolite of daidzein by the intestinal microflora, shows the strongest estrogenic activity among isoflavones.35,36 Daily isoflavone intake among non-Asian populations is estimated to be less than 3 mg whereas among older Japanese and Shanghai Chinese it is approximately 40 mg or more. According to the field survey, the consumption of isoflavones of Japanese people is about 20-40 mg per day, which is markedly higher than that of flavonoids except for tea catechins of 15 mg and carotenoids.31,35,36
Feeding the world sustainably has become important because, in half of the deaths that occurred in the six continents, the cause was an unhealthy diet.1–5 The food diversity, nutrient profile and glycemic index of our designer food designer food were acceptable because it has no adverse impact on the intake of salt, sugar and trans fat which are an important consideration for a healthy diet. It is clear that this designer food with these food qualities may be advised for prevention of diabetes mellitus and cardiovascular diseases (CVDs) (Table 1).Recently, many experts suggest a substantial reduction in red meat and refined and sweetened food intake and shifting toward plant-based dietary patterns.37–39 This is important to meet the unprecedented challenge of feeding a healthy and sustainable diet to an estimated 10 billion people by 2050.39 It seems that technological innovations are vital for creating this system. However, it may be wise to remain vigilant to ensure that these new products are beneficial to human health and diseases, as well as for the health of the planet.39–42 A recent study also reported the glucose-lowering effect of foxtail millet in subjects with impaired glucose tolerance.40 Similar beneficial effects of millets were reported from East Africa, among patients with diabetes.41 It is also wise to include medical rice in this formulation because it has a better nutrient profile and proven efficacy.33 However, millets have been found to protect against under nutrition in school going children.42
In brief, the findings of this real-world trial revealed that designer food supplementation was associated with a significant decline in fasting and postprandial blood glucose, total cholesterol and triglycerides along with oxidative stress and pro-inflammatory cytokines that are important coronary risk factors in patients with diabetes. We also found a significant increase in hemoglobin and calcium as well as magnesium indicating that this designer food can prevent under nutrition and anaemia as well as possibly osteoporosis. In view of these findings in our study, a designer food (whole food flour) consisting of millets (60%), soybean (20%), brown rice (10%), peanuts(8%), flax seeds(1-2%) in the form of bread, roasted bread in oil (paratha), roasted vegetables covered with designer flour(pakora), cakes and modern breads may be used for its beneficial effects in patients with type 2 diabetes. Since millets are nutritionally superior to other grains because they contain a high amount of proteins, flavonoids, carotenoids, magnesium, calcium and iron etc, they could be added as a functional food in other foods, for prevention of NCDs. Millets could also be used to combat micronutrient malnutrition by bio-fortification of staple crops. Millets could be mixed in cakes, biscuits, cookies, bread and pastries to provide proteins and above-mentioned micronutrients which may reduce the intake of refined and sweetened foods as well as red meat to provide functional foods.
The analysis of data is made from real world data obtained from hospital records. Informed consent from all the subjects was taken before advising diet. All subjects were included in this analysis, after permission from Halberg Hospital and Research Institute Ethics committee, Moradabad, India.
Logistic funds were provided by the International College of Nutrition for finding out the cytokines in poor patients.
All the data were collected by Dr RBS, others Dr JF,VM,AI,NR,MD,SW helped in writing of the manuscript.
All subjects gave informed consent for participation in the study. The study is based on real world data drawn from registry of Halberg Hospital and Research Institute, compliance with ethical standards not required.
Acknowledgements are given to Center of Nutrition Research, International College of Nutrition and International College of Cardiology for providing logistic support to conduct this study.
Conflicts of interest have not been reported by any of the authors.

©2020 Singh, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.