MOJ
eISSN: 2379-6383


Research Article Volume 6 Issue 6
1Halberg Hospital and Research Institute, India
2 Pavol Jozef Safaric University, Slovakia
3Dia-care and Hormone Institute, India
4Babu Banarasi Das University, India
5KG Medical University, India
Correspondence: Ram B Singh, MD, Halberg Hospital and Research Institute, Moradabad, India, Tel 91 999794102
Received: October 29, 2017 | Published: December 20, 2017
Citation: Singh RB, Fedacko J, Saboo B, et al. Association of higher Omega-6/Omega-3 fatty acids in the diet with higher prevalence of metabolic syndrome in north India. MOJ Public Health . 2017;6(6):456-464. DOI: 10.15406/mojph.2017.06.00193
Background: The epidemic of obesity and hypertension over the last two decades, in the middle and high income countries is associated with marked rise in the incidence of metabolic syndrome
Objective: To measure the prevalence of metabolic syndrome (MS) and determine its association with ratio of omega-6/omega-3 fatty acids in the diet.
Design and Methods: Cross-sectional surveys were conducted in 20 urban streets in the city of Moradabad, India. Randomly selected subjects with MS aged 25 years and above were evaluated and graded according to omega-6/omega-3 ratio in the diet. Physical examination, sphygmomanometer, questionnaire and blood tests were done.
Results: The overall prevalence of MS was 19.3% (n=387) and hypertension 26.6% (n=533) without any gender difference. The prevalence of MS, type 2 diabetes, CAD and hypertension showed a trend of higher rate, in relation to omega-6/omega-3 ratio in the diet. Subgroup analysis showed that subjects eating low omega-6/omega-3 ratio (<5.0) diets had significantly lower prevalence of MS, and related components compared to higher ratio diets, among both sexes. Multivariate logistic regression analysis after adjustment of age showed, that hypertriglyceridemia (odds ratio 0.90 in men, 0.76 in women) was strongly (P<0.01) associated with MS. Hypertension, HDL-C, and central obesity were weakly associated with MS in both sexes. Hypercholesterolemia was weakly associated with MS only in women.
Conclusion: MS has become a public health problem in India. Higher w-6/w-3 ratio is a major risk factor of MS and CAD. It is possible that a low w-6/w-3 ratio in the diet (<5.0) may be protective against MS and related components.
Keywords: Nutrition; Alpha-linolenic acid; Food intakes; Risk factors; Hypertension; Coronary disease; Diabetes
The epidemic of obesity and hypertension over the last two decades, in the middle and high income countries is associated with marked rise in the incidence of metabolic syndrome (MS), CAD, type 2 diabetes, myocardial infarction and stroke and the total burden of cardiovascular disease (CVD) [1-4]. The metabolic syndrome is associated with constellation of metabolic disturbances, of all the risk factors for CVD, [4]. MS appears to be a major cause of mortality and morbidity due to CVD and diabetes [5-9]. In the 1920s, Kylin, a Swedish physician, described MS, as the clustering of hypertension, hyperglycemia, and gout [10]. However, the concept of the MS has existed for at least 80 years [4]. In 1947, Vague [11], drew attention to upper body adiposity (android or male-type obesity) as the obesity phenotype that was commonly associated with metabolic abnormalities characterized with type 2 diabetes and CVD. There is urgent need for strategies to prevent the emerging global epidemic, as this syndrome appears to be a master of disguise since it can present in various ways according to the various components that constitute the syndrome [3-9]. Reaven [12], described the MS as syndrome X, De Fronzo et al. [13], as the insulin resistance syndrome, and Kaplan [14], the deadly quartet. The MS represents a constellation of metabolic abnormalities including glucose intolerance (type 2 diabetes, impaired glucose tolerance, or impaired fasting glycaemia), insulin resistance, central obesity, dyslipidemia, and hypertension, which are well known risk factors for CAD [2,3]. Epidemiological studies suggest that primary risk factors such as physical inactivity and unbalanced nutritional consumption of excess calories, simple refined carbohydrates with a high glycemic index and load of high saturated fat (SF), trans fatty acids (TFA), and high w-6/w-3 ratio and lower monounsaturated fatty acids, in the diet are pro-inflammatory [8-15], and contribute to the escalating rates of obesity, MS and mortality due to CVD [1-16].
In the 5th century (BCE), Confucius, the Chinese philosopher taught his students, “Cereals, the basic, fruits the subsidiary, meat the beneficial and vegetable the supplementary”. Therefore, the concept of eating a diet high in animal foods, and preference for meat, possibly wild animals and birds rich in w-3 fatty acids, and whole grain cereals have been shaped over hundreds of years among Chinese. While Greek physician Hippocrates (600 BCE) advocated food as medicine, adverse effects of Tamasic foods characteristics of the Western diet, were proposed by Indian ancient physicians; Charak and Sushruta in 600 BCE. Charaka, who was Brahmin, supposed to live in Taxila University in North of India, proposed that “Heart attack is born by the intake of fatty meals, overeating, excess of sleep, lack of exercise and anxiety” (Charaka Sutra, 600BC). Sushruta was a surgeon from Vishwamitra family from Varanasi, in East India who was in a position to do surgery and described atherosclerosis as madroga; “Excess intake of fatty foods and lack of exercise causes obesity and narrowing of the channels taking blood to the heart. It is useful to use guggul, triphala and silajit in the treatment”. The total fat and saturated fat intake as a percentage of total calories has continuously decreased in Western diets in the last 40 years, whereas the omega-6 fatty acid has increased and the omega-3 fatty acid decreased, resulting in a large increase in the omega-6/omega-3 ratio from 1:1 during evolution to 20:1 today in the western world [7-9]. The ratio of omega-6 and omega-3 fatty acids has markedly increased to 45:1 among South Asians, due to marked increase in the intake of sunflower, corn oil and soya bean oils [8,9]. These dietary changes in the composition of fatty acids are associated with significant increase in the prevalence of obesity, hypertension and metabolic syndrome [17]. In the present study, we examine the association of w-6/w-3 ratio of diet with risk of MS and its other components; hypertension, CAD and dyslipidemia.
Selection of subjects
We randomly selected 20 streets from the urban area of the city of Moradabad. From each street; blocks or clusters were randomly selected, and from each block, 40-100 adults, aged 25 years and above were randomly selected based on voter’s list. When the random number fell to a subject, who was <25yrs or not available, it was assigned to next person in the list. We contacted 2422 urban subjects aged 25 yrs and above, of which 220(9.08%) refused to participate and rest 2002(1016men and 986 women volunteered to be included in the study. Detailed interviews were performed with the help of pretested and validated questionnaire, prepared according to the guidelines of WHO and Indian Council of Medical Research. Dietary assessment was made by 7-day food intake record by questionnaires. Evaluation was by a physician and dietitian administered questionnaire, a physical examination and sphygmomanometer and blood tests. The diagnosis of metabolic syndrome was based on WHO criteria (presence of 3 risk factors or more; hypertension, central obesity, type 2 diabetes or glucose intolerance), and subjects were graded according to w-6/w-3 ratio of fatty acids, in the diet.
Criteria
Body mass index was calculated and obesity was defined as a body mass index >30 kg/m2 and above, overweight when body mass index >25 kg/m2 and up to 29.9. Figures for criteria according to the Indian consensus group for overweight (>23 kg/m2), were also calculated. Central obesity was considered when waist- hip ratio >0.90 in males and >0.85 in females were observed, as suggested in previous studies [1,2]. Diabetes mellitus was diagnosed in presence of fasting blood glucose >7.7 mmo1/1 (140 mg/dl) and postprandial 2 h after 75 g of oral glucose >11.2 mmol/1 (>200 mg/dl). Glucose intolerance was diagnosed in presence of fasting glucose between 110-140mg/dl and post prandial glucose between 180 to 200mg/dl. It is difficult to assess tobacco intake, because it is consumed in various forms. Cigarettes, beedies, Indian pipes, raw tobacco and chewing tobacco are all commonly consumed and people use tobacco in more than one form .We therefore categorized users of any form of tobacco as smokers as was done in previous studies. Individuals who admitted to ingesting alcohol more than once a week were categorized as alcohol consuming. Blood pressure was measured in right arm (systolic and diastolic phase V of Korotkoff) after 5 min rest in sitting position according to WHO guidelines by a single mercury manometer and by the same physician in all subjects . Hypertension was diagnosed when systolic blood pressure was 140 mm Hg or more and diastolic blood pressure 90 mmHg or more. Nutrient intakes were calculated with the help of nutrient composition of Indian foods tables [18].
The criteria for the diagnosis of CAD were:
Presence of all of these three criteria was taken as confirmation of the diagnosis of CAD. Individual clinical criteria included known CAD, affirmative response to Rose questionnaire and electrocardiographic changes (Q wave change codes 1-1 and 1-2, ST segment depression or elevation codes 4-1, 4-2 and 9-2 and T wave inversions, codes 5-1 and 5-2. Prevalence rates of these electrocardiographic finding with and without clinical criteria for CAD are also given. A blood sample after an overnight fast was obtained from all subjects. Each participant was asked to drink 75 g anhydrous glucose in 200 ml of water and a second blood sample was collected after 2 h for analysis of glucose.
The prevalence rates are given in percent and numerical variables as mean 1 standard deviation. Significance of association of various risk factors was determined by multivariate logistic regression analysis. Odds ratios and 95% confidence intervals were calculated by multivariate analysis after adjustment of age and sex using overall prevalence of metabolic syndrome, as the dependent variable. Subjects were classified based on n-6/n-3 ratio in the diet, and the association of various components of metabolic syndrome was demonstrated by Mantel Haenzel Chi square test and Kendal’s t test.
We studied 2002 subjects 25years and above, from North India. The age and sex distribution of the sample were comparable with the age and sex ratio in the population of Uttar Pradesh. Table 1 shows the fatty acid intakes in the urban population studied compared to a rural population of the same district [19]. The Consumption of total, saturated fat, polyunsaturated fat and linoleic acid were slightly higher among men compared to women (Table 1). The consumption of alpha-linolenic acid which is a short chain w-3 fatty acid was also higher in men compared to women. The ratio of w-6/w-3 fatty acid showed no significant difference among sexes. Table 2 shows the prevalence of coronary risk factors and metabolic syndrome in our subjects. The prevalence of coronary artery disease, diabetes mellitus, low HDL and tobacco intake were significantly higher among men compared to women. The overall prevalence of metabolic syndrome was 19.3% without any sex difference. Table 3 shows the prevalence of coronary risk factors, CAD, MS and its components in relation to w-6/w-3 ratio in the diet. This table shows that there was an overall increase in the prevalence of CAD, type 2 diabetes, hypertension, hypertriglyceridemia (>150mg/dl), low HDL, central obesity and metabolic syndrome among subjects consuming high w-6/w-3 ratio diet and the trend was significant for both men and women which is better depicted in the Figure 1. An increasing ratio of w-6/w-3 ratio in the diet was also associated with a rising trend in mean levels of body mass index, waist-hip ratio, blood pressures, Triglycerides, HDL cholesterol and fasting blood glucose and the trends were significant (Table 4). Table 5 shows that there was a significant positive rank correlation between the level of w-6/w-3 fat ratio in the diet and components of metabolic syndrome and coronary risk factors; mean age, body weight, body mass index, waist-hip ratio, systolic and diastolic blood pressures, total cholesterol, triglycerides, and fasting blood glucose. Multivariate logistic regression analysis showed that regardless of age, in relation to w-6/w-3 ratio in the diet, hypertriglyceridemia, HDL cholesterol, hypertension, central obesity, physical activity, fasting blood glucose were significantly associated with metabolic syndrome among both sexes. Hypercholesterolemia was not associated with MS among men.
Urban |
||
Women |
Men |
|
Total energy % k cal/day |
2047 |
2280 |
Total fatty acids (g/day) |
60.0±6.8 |
64.5±7.6 |
Saturated fat (g/day) |
20.5±4.2 |
22.0±5.3 |
Monounsaturated (g/day) |
22.5±4.6 |
24.5±5.5 |
Polyunsaturated (g/day) |
17.0±4.1 |
18.0±4.4 |
Linoleic acid (g/day) |
16.5±3.8 |
17.4±4.0 |
Alpha-Linolenic acid (g/day) |
0.510.11 |
0.64±0.11 |
Omega-6/Omega-3 |
33.0±5.6 |
29.0±5.2 |
Polyunsaturated/Saturated fat ratio |
0.82±0.2 |
0.82≥±0.2 |
Prevalence of coronary artery disease (CAD)(n (%)) |
67(6.8) |
119(11.7) |
Table 1: Fatty acid consumption in urban subjects.
* = P <0.01, Total CAD among both sexes. 9.3%
Risk factor |
Men (n=1016) |
Women (n=986) |
Total (n=2002) |
Coronary artery disease |
119(11.7)* |
67(6.8) |
186(9.3) |
Diabetes mellitus |
81(8.0)* |
52(5.3) |
133(6.6) |
Hypertension (>140/90) |
285(28.0) |
248(25.1) |
533(26.6) |
Hypercholesterolemia (>55.18mm) hypertriglyceridemia |
313(30.8) |
317(32.1) |
630(31.4) |
Low high density |
335(33.0) |
345(35.0) |
680(33.9) |
lipoprotein cholesterol (<1.04mmd) |
275(27.0)* |
146(14.8) |
421(21.0) |
Central obesity (>0.90 men, >0.85 women) |
482(47.4) |
506(51.3) |
1007(50.3) |
Metabolic syndrome |
202(19.8) |
185(18.7) |
387(19.3) |
Tobacco intake |
202(19.8)** |
18(1.8) |
220(10.9) |
Obesity (BMI>25Kg/m2) |
321(31.6) |
324(32.8) |
645(32.2) |
Table 2: Prevalence of coronary risk factors and metabolic syndrome.
*=P<0.05 by Chi square test
W-6/W-3 Ratio |
No. of Subject |
CAD |
BP >140/90mmHg |
Diabetes |
Total triglycerides >1.69 mmol/L |
HDL <1.04mmol/L |
Cen.Obe WHR >0.90 |
Metabolic Syndrome |
Men (n=1016) |
||||||||
< 5.0 |
230 |
8(3.5) |
19(8.2) |
7(3.0) |
21(9.1) |
14 (6.0) |
25(10.8) |
5(2.3) |
5.1-10.0 |
542 |
53(9.7) |
170(31.3) |
44(8.1) |
188(34.7) |
164(30.2) |
277(51.1) |
100(18.4) |
>10.0 |
244 |
58(23.7) |
96(39.4) |
30(12.3) |
126(51.6) |
97(39.7) |
170(69.6) |
97(39.7) |
Total |
1016 |
119(11.7) |
285(28.0) |
81 (7.9) |
335(32.9) |
275(25.9) |
482(47.4) |
202(19.8) |
Mantel Haenszel |
x2 |
29.71 |
32.25 |
18. 4 |
25.64 |
28.5 |
33.62 |
19.62 |
P Value |
<0.001 |
<0.001 |
<0.01 |
<0.001 |
<0.001 |
<0.001 |
<0.01 |
|
Women(n=986) |
||||||||
< 5.0 |
233 |
6(2.6) |
31(12.8) |
5(2.1) |
29(12.4) |
7 (3.0) |
28(12.0) |
4(1.7) |
5.1-10.0 |
515 |
31(6.0) |
136(26.4) |
29(5.6) |
178(34.5) |
81(15.7) |
275(53.4) |
91(17.6) |
>10.0 |
238 |
30(12.6) |
81(34.0) |
18(7.5) |
138(58.0) |
48(20.1) |
203(85.3) |
90(37.8) |
Total |
986 |
67(6.8) |
248(25.1) |
52(5.3) |
345(35.0) |
146(14.8) |
506(51.3) |
185(18.6) |
Mantel Haenszel |
x2 |
30.52 |
28.81 |
19.62 |
33.41 |
22.44 |
29.71 |
17.63 |
P Value |
<0.001 |
<0.001 |
<0.002 |
<0.001 |
<0.002 |
<0.001 |
<0.01 |
|
Table 3: Prevalence of coronary risk factors in relation to omega-6/ omega-3 ratio of diet.
HDL: High Density Lipoprotein Cholesterol; WHR: Waist-Hip Ratio; CAD: Coronary Artery Disease.
w-6/w-3 |
n |
BMI (Kg/M2) |
WHR |
BP (mmHg) |
Triglycerides (mmol/L) |
HDL-C (mmol/L) |
Fasting Blood Glucose (mmol/L) |
|
Systolic |
Diastolic |
|||||||
Men (n=1016) |
||||||||
>5.0 |
230 |
20.2(3.2) |
0.87(0.10) |
119.0(13) |
78.5(10) |
1.27(0.5) |
1.18(0.3) |
4.6(1.0) |
5.1-10.0 |
542 |
22.4(4.1) |
0.89(0.12) |
125.1(16) |
84.6(12) |
1.68(0.7) |
1.17(0.3) |
5.0(1.1) |
>10.0 |
244 |
24.5(4.3) |
0.92(0.13) |
131.6(18) |
88.2(14) |
1.98(0.8) |
1.13(0.2) |
5.4(1.2) |
Total |
1016 |
22.4(4.2) |
0.89(0.11) |
125.3(17) |
84.1(13) |
1.66(0.7) |
1.16(0.3) |
5.0(1.1) |
Kendal’s t-test |
0.047 |
0.98 |
0.105 |
0.091 |
0.035 |
0.031 |
0.037 |
|
T Value |
2.75* |
4.8** |
4.57** |
4.1** |
2.43* |
1.45 |
2.58* |
|
Women (n=986) |
||||||||
>5.0 |
233 |
20.0(3.1) |
0.83(0.09) |
21(14) |
80.2(11) |
1.60(0.5) |
1.25(0.5) |
4.5(1.0) |
5.1-10.0 |
515 |
22.2(3.5) |
0.86(0.11) |
124(16) |
85.5(13) |
1.66(0.7) |
1.22(0.4) |
5.1(1.1) |
>10.0 |
238 |
24.7(4.0) |
0.89(0.12) |
130(17) |
89.0(14) |
1.70(0.8) |
1.17(0.2) |
5.4(1.2) |
Total |
986 |
22.3(3.7) |
0.86(0.13) |
125 (18) |
85.1(15) |
1.66(0.7) |
1.22(0.5) |
5.0(1.1) |
Kendal’s t-test |
0.049 |
0.91 |
0.103 |
0.089 |
0.037 |
0.033 |
0.039 |
|
T Value |
2.81* |
4.6** |
4.98** |
3.99** |
2.65* |
1.47 |
2.59* |
|
Table 4: Risk factor levels in relation to omega-6/omega-3 fat ratio in the diet.
P value *= <0.01, **=<0.001
This study shows that various components of MS; hypertension, central obesity, HDL cholesterol, hypertriglyceridemia, fasting blood glucose were significantly associated with MS in relation to w-6/w-3 fatty ratio in the diet (Table 5 & 6). People of south Asian origin wherever they are settled have an increased vulnerability to coronary artery disease (CAD) and type 2 diabetes, compared to indigenous populations [1-20]. The cause of increased susceptibility of south Asians to CAD and diabetes may be due to MS [20,22]. However, there are no large scale population based studies among South Asians indicating prevalence of MS in South Asia. The prevalence of MS in our study was 19.3% (n=387) including 19.8% among men and 18.7% among women. MS has been observed in many ethnic groups and it is estimated that it is prevalent in approximately one fourth of the adult population of the world [7-21]. In a multicenter case control study from India, involving 5088 subjects with known type 2 diabetes, the overall prevalence of MS was 77.2%, and the rates were significantly higher among women compared to men, respectively (87.7 vs. 69.3%, P<0.0001) [20]. Important components of MS in this study were, hypertension, followed by hypertriglyceridemia in men and central obesity followed by hypertension in women. In another study from India, among 1806 urban subjects, aged 25-64 years, the prevalence of type 2 diabetes mellitus was 6.0%, hypertension 24.0% and CAD 9.0% [22]. Among subjects with diabetes, the prevalence of central obesity was 95.3%, CAD 23.5% and hypertension 51.6%, which are lower compared to prevalence of these components in Mumbai, West India. These differences in risk factors may be explained by the differences in diet and lifestyle factors in West and North India [23].
Men |
Women |
|||
Mean |
R |
Mean |
R |
|
Mean age (years) |
53.5(13) |
0.06 |
51.6(11) |
0.04 |
Mean body weight (Kg.) |
64.0(07) |
0.08* |
52.5(4) |
0.09* |
Body mass index (>25Kg./m2) |
25.5(05) |
0.08* |
22.4(4) |
0.09* |
Waist hip ratio |
0.90(0.2) |
0.08* |
0.86(0.2) |
0.07* |
Systolic blood pressure(mmHg) |
130(21) |
0.21** |
128(19) |
0.19** |
Diastolic blood pressure(mmHg) |
88(16) |
0.78** |
84(14) |
0.16** |
Total cholesterol (mmol/L) |
5.12(1.1) |
0.09* |
5.0(1.1) |
0.08* |
Triglycerides (mmol/L) |
1.67(0.06) |
0.04 |
1.69(0.07) |
0.07* |
High density lipoprotein Cholesterol(mmol/L) |
1.17(0.04) |
-0.05 |
1.21(0.04) |
-0.04 |
Fasting blood glucose (mmol/L) |
5.1(1.2) |
0.07* |
5.1(1.1) |
0.08 |
Table 5: Mean levels of clinical and biochemical risk factors and their correlation with omega-6/omega-3 fat ratio in the diet (Spearman’s rank correlation).
Risk Factors |
Odds Ratio |
Men (95% CI) |
Odds Ratio |
Women(95% CI) |
Hypertriglyceridemia |
0.9 |
(0.81 to 0.98)** |
0.76 |
(0.69 to 0.83)** |
HDL cholesterol |
0.69 |
(0.62 to 0.78)* |
0.72 |
(0.67 to 0.79)* |
Hypertension |
0.91 |
(0.82 to 0.98)* |
0.67 |
(0.61 to 0.78)* |
Central obesity |
0.83 |
(0.76 to 0.92)* |
0.86 |
(0.80 to 0.92)* |
Physical activity |
0.81 |
(0.68 to 0.97)* |
0.46 |
(0.37 to 0.51)* |
Hypercholesterolemia |
0.92 |
(0.82 to 1.09) |
0.78 |
(0.71 to 0.87)* |
Fasting blood glucose |
0.84 |
(0.78 to 0.95)** |
0.81 |
(0.75 to 0.91)* |
Table 6: Age-adjusted Odds Ratio and confidence intervals for association of risk factors with metabolic syndrome in relation to omega-6/omega-3 fat ratio by logistic regression analysis.
Rural |
Urban |
|||
Women |
Men |
Women |
Men (n=1016) |
|
Total energy % k cal/day |
2035 |
2267 |
2047 |
2280 |
Total fatty acids (g/day) |
35.8±4.8* |
37.6±5.1* |
60.0±6.8 |
64.5±7.6 |
Saturated fat (g/day) |
11.8±3.4* |
12.5±3.6* |
20.5±4.2 |
22.0±5.3 |
Monounsaturated (g/day) |
13.5±3.5* |
14.6±3.7* |
22.5±4.6 |
24.5±5.5 |
Polyunsaturated (g/day) |
10.5±2.4* |
10.5±2.5* |
17.0±4.1 |
18.0±4.4 |
Linoleic acid (g/day) |
9.3±2.5* |
9.2±2.6* |
16.5±3.8 |
17.4±4.0 |
Alpha-Linolenic acid (g/day) |
1.2±0.3* |
1.30±.3* |
0.5±0.11* |
0.6±0.11* |
Omega-6/Omega-3 |
7.7±1.6* |
7.0±1.5* |
33.0±5.6* |
29.0±5.2* |
Polyunsaturated/Polyunsaturated |
0.88±0.2 |
0.84±0.2 |
0.82±0.2 |
0.82±0.2 |
Prevalence of coronary artery disease (CAD)(n (%)) |
27(2.83)* |
40(4.06)* |
67(6.8) |
119(11.7) |
Table 7: Fatty acid consumption in rural and urban subjects.
* = P¬ <0.01, Total CAD among both sexes. 3.4%* 9.3%
In another study among Indian Immigrants to USA [24], the prevalence of MS was 77.0%. These studies indicate an increased susceptibility of Indians to MS, possibly leading to type 2 diabetes and CAD. The prevalence of CAD and diabetes are approximately 3.0% in rural, 10.0% in urban and 14.0% in south India and immigrants to developed countries [19-23]. The w-6/w-3 ratio of the diet is 5/1 to 7/1 in rural, 30/1 to 50/1 in urban subjects, indicating a gradient in the risk with increase in w-6/w-3 fatty acids ratio in the diets (Table 1) [9-25]. We observed a high prevalence of CAD, MS and its components in association with increasing ratio of w-6/w-3 fat in the diet and the trends were significant both among men and women (Tables 2-4) (Figure 2). The five city study showed a greater prevalence of CAD in Mumbai (staple oil ground nut), than Kolkata, east India and Moradabad north India who consume mustered oil rich in w-3 fatty acids [1-23]. The Indian Lifestyle and Heart Study showed, significantly lower prevalence of CAD (11.0 v/s 13.5 and 16.2 %, P<0.02) compared to subgroups, eating Indian ghee and vegetable ghee+ linoleic acid(w-6), respectively, among subjects eating, moderate to heavy fat diets [26]. The Prevalence of CAD (3.0 v/s 7.9,18.2%,P<0.001), type 2 diabetes (2.6 v/s 6.9 and 9.9%, P<0.02) and metabolic syndrome (1.9 v/s 18.0 and 38.8%, P<0.01) were significantly lower in the subgroup (n=466) consuming low w-6/w-3 ratio diet (<5.0) compared to subgroups eating higher ratios diets (5.1/1 to 10.0/1, n=1057) and >10.0/1 (n=482). These subgroups also had greater body fat measured by bioelectrical impedance analysis, despite low rates of obesity [27].
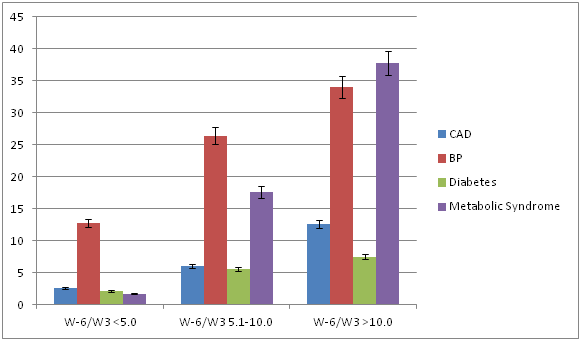
Figure 2: Prevalence of coronary risk factors in relation to omega-6/ omega-3 ratio of diet in women.
We observed a higher prevalence of CAD, hypertension, diabetes mellitus, hypertriglyceridemia, central obesity and MS and high prevalence of low HDL among subjects consuming high w-6/w-3 ratio(5.0-10.0 and >10.0) diet compared to subjects taking low w-6/w-3 ratio(<5.0) diet among both sexes and the trends were significant (Table 3). The levels of mean systolic and diastolic blood pressures, serum triglycerides, and fasting blood glucose were significantly greater among subjects receiving high w-6/w-3 ratio diets, compared to those receiving low w-6/w-3 ratio diets in both men and women (Table 4). We also observed a significant positive rank correlation between w-6/w-3 ratio in the diet and body mass index, waist/hip ratio, blood pressures and fasting blood glucose (Table 5). There are no population based studies from India regarding association of CAD, MS and diabetes with w-6/w-3 ratio in the diet; hence we cannot compare our results with other studies. Deficiencies of EPA and DHA have been observed in subjects of south Asian origin, living in UK. Despite adequate intake of ALA (1.0 to 1.6mg/day) in the diet, this may be due to decreased delta-6, delta-5 desaturase enzyme activity, responsible for conversion of ALA to EPA and DHA or their increased consumption in the tissues [9]. There is evidence that green leafy vegetables, whole grains, walnuts, flex seeds and canola oil or mustered oil are rich in alpha-linolenic acid (w-3 fatty acid) and Mediterranean diet or Indo-Mediterranean which is rich in these foods may be protective against cardiovascular disease [28-35].
In the Lyon diet heart study [33], 605 patients with post myocardial infarction were randomly assigned to Mediterranean style diet or control diet resembling National Cholesterol Education program step 1 diet. The Mediterranean diet supplied more than 0.6% energy from alpha-linolenic acid and <10% from saturated fatty acid, out of 30% energy from fats. After a mean follow up of 27 months, the risk of new AMI and episodes of unstable angina were reduced by 70% in the Mediterranean diet group compared to control group. The Indo-Mediterranean Diet Heart Study was a randomized, single blind trial, conducted among 1000 patients with high risk of recurrent cardiac events [32]. Half of the patients (n=499) were administered a diet rich in whole grains, fruits, vegetables, walnuts and mustered or soya bean oil as a source of alpha-linolenic acid (ALA, w-3), and 501 patients were advised to consume prudent diet. After 2 years of follow up, the intervention group received two fold greater ALA compared to control group (1.8 vs. 0.8g/day) resulting into marked decline in the w-6/w-3 ratio in the two diets (mean SD 9.1+12 v/s 21+10, p<0.001), respectively. Total cardiac events were significantly fewer, in the intervention group, than in the controls (39 v/s 76 events, p<0.001). Sudden cardiac deaths were also decreased (6 v/s 16, p<0.015), as were nonfatal infarction (21 v/s 43, p<0.001). These findings indicate that dietary changes may alter w-6/w-3 ratio, which may be associated with large reduction in CAD risk. Further benefit may be observed, if soya bean oil is avoided by using more traditional mustered oil in the Indian diets, as observed in the rural Indian diets (Table 1). In a more recent study [36], Esposito et al randomized 180 patients with MS to a Mediterranean style diet or a step 1 diet with fat intake <30.0%. After 2 years, intervention group showed greater weight loss, had lower C-reactive proteins and other pro-inflammatory cytokines levels , less insulin resistance, lower total cholesterol and triglycerides and higher HDL-cholesterol levels and had a 50% decrease in the prevalence of MS. This study confirms that Mediterranean diet provide beneficial effects on inflammation, dyslipidemia as well as decrease insulin resistance and improve endothelial function in patients with MS.
Recent studies indicate that w-3 fatty acids appears to be important in the pathogenesis of acute coronary syndrome and its complications; arrhythmias, heart failure and cardiac events [37-39]. Further evidence indicate that total fat, saturated fat and trans fat can enhance inflammation and visceral obesity resulting in to MS and treatment with w-3 fatty acids may be beneficial [40-42]. In a recent study, a total of 117 volunteers completed the 12-week trial. Participants in the 1-, 3-, and 6-portions/d groups reported consuming on average 1.1, 3.2, and 5.6 portions of fruit and vegetables, respectively, and serum concentrations of lutein and β-cryptoxanthin increased across the groups in a dose-dependent manner [43]. For each 1-portion increase in reported fruit and vegetable consumption, there was a 6.2% improvement in forearm blood flow responses to intra-arterial administration of the endothelium-dependent vasodilator acetylcholine (P=0.03). There was no association between increased fruit and vegetable consumption and vasodilator responses to sodium nitroprusside, an endothelium-independent vasodilator. In an earlier randomized trial in patients with high risk of CVD, two third had MS, supplementation with fruits, vegetables, whole grains and nuts was protective against risk factors; dyslipidemia, hyperglycemia and oxidative stress which are components of MS [44]. Omega-3 fatty acids can regulate leptin gene expression and the concentrations of anandamides in the brain, which in turn binds to endogenous cannabinoid receptors and regulate food intake and satiety as well as weight gain [9]. Deficiency of w-3 fat can increase appetite, resulting into obesity and MS.
CVD, diabetes mellitus, cancer, autoimmune diseases, rheumatoid arthritis, asthma and depression are associated with increased production of thromboxane A2, leucotrienes, interleukins-1 and 6, tumor necrosis factor-alpha and C-reactive proteins [31-35]. Increased dietary intake of w-6 fatty acids without consideration for w-3 fat is known to enhance all these risk factors as well as atherogenicity of cholesterol and oxidized LDL cholesterol which have adverse pro-inflammatory effects and may result into thrombosis and acute coronary syndrome (ACS), cancer, diabetes mellitus and metabolic syndrome. Omega-3 fatty acids are known to reverse all these biochemical adverse effects hence a low w-6/w-3 ration of 1:1 has been suggested in the Columbus concept [17]. Although, most workers working on dietary patterns do not mention the nutrient content of their prudent diet, but one single difference is the w-3 fatty acid, apart from other micronutrients, which is rich in fruits, leafy vegetables, nuts and whole grains. It would be very interesting to know the role of refined starches and sugar, large meals, decreased intake of fruits, vegetables, whole grains and nuts on inflammation and endothelial function and nitric oxide levels as risk predictors of MS and its components [44-51]. Fruits, vegetables, nuts, whole grains, animal foods rich in w-3 fatty acids are slowly absorbed and may prevent the increase in free fatty acids, and inflammation, which is a characteristic of MS.
There is evidence that omega-6 and omega-3 fatty acids elicit divergent effects on body fat gain through mechanisms of adipogenesis, browning of adipose tissue, lipid homeostasis, brain-gut-adipose tissue axis, and most importantly systemic inflammation [53-56]. Prospective studies clearly show an increase in the risk of obesity as the level of omega-6 fatty acids and the omega-6/omega-3 ratio increase in red blood cell (RBC) membrane phospholipids, whereas high omega-3 RBC membrane phospholipids decrease the risk of obesity. Recent studies in humans show that in addition to absolute amounts of omega-6 and omega-3 fatty acid intake, the omega-6/omega-3 ratio plays an important role in increasing the development of obesity via both AA eicosanoids metabolites and hyperactivity of the cannabinoid system, which can be reversed with increased intake of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). A balanced omega-6/omega-3 ratio in the diet, in conjunction with reduction in refined carbohydrates and saturated fat are important for health and in the prevention and management of obesity, metabolic syndrome and other chronic diseases [57-59].
This study reveal that high w-6/w-3 ratio in the diet appears to be a risk factor of MS and its components; hypertension, hypertriglyceridemia, hyperglycemia, low HDL and central obesity. Our findings validate that increased intake of w-3 rich foods and lower intake of w-6 fatty acids with low w-6/w-3 fatty acid ratio in the diet may be protective against MS. Further studies are necessary to confirm our findings.
We would like to thank International College of Nutrition and Sandoz Foundation of Gerontologic Research for providing financial support to conduct this study.
None.

©2017 Singh, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.