MOJ
eISSN: 2379-6383


Research Article Volume 9 Issue 5
1College of Medical Radiologic Science, Sudan University of Science, Sudan
2College of Applied Medical Sciences, Qassim University, Saudi Arabia
3Alghad International Medical Sciences College, Saudi Arabia
4College of Radiography & Medical Imaging Sciences, National University, Sudan
Correspondence: Mohammed Ahmed Ali Omer, College of Applied Medical Sciences, Qassim University, Buraidah, Qassim, Saudi Arabia, Tel +966508037217
Received: September 02, 2020 | Published: September 30, 2020
Citation: Sakin GAE, Gar-elnabi MEM, Omer MAA, et al. Assessment of effects induced by bone scintigraphy dose in red & white blood cells relative to ageing and obesity. MOJ Public Health . 2020;9(5):160-164. DOI: 10.15406/mojph.2020.09.00340
Introduction: The indispensable Nuclear Medicine (NM) technology has been utilized for diagnosis of pathologies, cancer staging and researches; however, it accompanied by potential hazards; hence the aim of this study was to estimate the impact of radioactive dose of bone scintigraphy in quantity of WBCs and RBCs relative to ageing and body mass index (BMI).
Methods: The Technetium-99m generator eluted based on time activity formula ( ) and further the eluted dose mixed with Methylene Diphosphate (MDP) and injected to specific patient based on BMI. Then WBCs and RBCs before and after 3-3.5 hours of dose injection estimated using automated cell counter (Sysmex KX-21) and the collected data of 150 patients analyzed by SPSS.
Results: The results showed that Females were the common gender referred to bone scan representing 70%. The WBC and RBCs count increases following the ageing from 18–61 and 18-50 years old respectively then decreases following ageing. WBCs increases as the BMI increases from≤18.49 (underweight) up to 25–29.9 (overweight) then decreases among obese (30 – 39.9) and peaking among Morbidly obese (≥ 40 Kg) but remains in normal range, while RBCs increases as the BMI increases from underweight (≤18.49) up to normal BMI (18.5–24.9) then persist semi constant in normal range among the rest weights. The activity dose (15±2.9 mCi) of bone scan reduces significantly the WBCs by 3.8% at P-value = 0.00 and P=value = 0.05) relative to age and BMI respectively but reduced RBCs insignificantly (P-value = 0.32) by 3.6% relative to normal.
Conclusion: NM doses of 15±2.9 mCi induce significant reduction in WBCs with regard to age and BMI, while the effect in RBCs was insignificant. Hence the ideal count of WBCs is necessary to be assessed before bone scan and the dose better reduced as less as the applied formula gave. Other methods of dose estimation will be contemplated and the image quality could be maintained by increasing image acquisition time or using SPECT.
Keywords: bone, scintigraphy, blood, irradiation, technetium-99m
Nuclear medicine imaging (NMI) as one of indispensable technology has been used for diagnosis of different pathologies, staging of cancerous diseases and researches, as it provides morphological anatomy, physiological information and even metabolic process.1,2 Despite its valuable benefits and low exposure dose; there have been potential radiation exposure hazards for both staff and patients that should be considered under radiation protection guides and rules to limit the stochastic effects.3
The common highlighted committed dose by radiation workers has been registered among western countries which was 5 mSv /year for interventional fluoroscopists (cardiologists and radiologists) that leading to cancer risk probability as 1/100 after incubation period of 20-30 years of work.4 Parallel to this fact, Chen et al,5 stated that: the radiology and nuclear medicine (NM) examinations account for mean effective dose of 3.0 mSv, while the patients underwent cardiac radiologic examination in USA received mean cumulative effective dose of 23.1 mSv over three years (range 1.5–543.7 mSv). A recent study of almost 1,000,000 non-elderly adults in healthcare markets across the United States showed that a considerable number of patients received up to 0.05 Sv/year; such considerable radiation dose, taken and indexed as reference levels that provided by International Commission on Radiation Protection (ICRP) in the range of 0.02 - 0.05 Sv/year.6,7 Accordingly, Nassef and Kinsara, et al.,8 showed that: the annual average effective doses for diagnostic radiology, nuclear medicine, and radiotherapy workers were 0.66, 1.56, and 0.28 mSv, respectively at King Abdul-Aziz University hospital in KSA.
The previous studies in this realm revealed that the effects of ionizing irradiation accompanied by symptoms and signs (diarrhea, nausea, vomiting, fatigue, infection and internal hemorrhage) within few minutes after exposure then followed by considerable reduction in blood components with immediate response in white blood cells (WBCs) and severe drop within 24 hours; which is dependent on quality of radiation, dose, dose rate, fractionation and the status of the object.9,10 While platelets decrease gradually by time.11 Also, El-Shanshoury et al,12 presented the significant reduction effects of blood components due to total body irradiation of Rats by Gamma radiation with doses of 0.1, 0.2, 0.3, 0.4, 0.5, 0.75 and 1.0 Gy.
Other relevant effects induced by radiation had been conducted by Abojassim et al,13 in which they showed the effect of gamma ray (Cesium-137 source with 5 µCi) at doses of 0.055 Gy, 0.11 Gy and 0.165 Gy on some hematological parameters of albino female rats (4 groups each 3 rats). Their results revealed that: there was significant (p≤0.05) decrease in the RBCs, Hb, Platelets, WBCs count, lymphocytes count, monocytic, neutrophils, eosinophiles and basophiles while MCV and MCH was increased and MCHC% did not change significantly. The effects of radiation dose in blood components also, have been highlighted by Eastlind & Charlonneau, et al,14 and they found that there were no significant differences in the dose response between isolated lymphocytes and granulocytes, whereas these cells had significantly more damage seen in whole blood lucokocytes and the neutrophils and they could tolerate a dose up to 175 Gy i.e. showed no change of aggregation while the granulocytes were appeared more radio-resistance than lymphocytes. In the same scope, Van, et al.,15 have revealed that a dose of 25-50 Gy would result in a reduction of monocytes growth and survival in isolated culture while the RBCs were shown to be most radio-resistance to -radiation relative to whole blood components.
The impact of ionizing radiation in medical field imaging exceeds the quantitative effects in blood components and furtherly involving the morphological changes. In this consent; Deyi Xu, et al,16 revealed the alterations in the ultrastructure of RBCs by electron microscope due to gamma irradiation doses (25–35 Gy). The changes seen were the concentrations of plasma electrolytes, lethality of lymphocytes, in addition to echinocytes, sphero-echinocytes (spiked RBCs) and erythrocytes with a degenerated shape.
The trend of this study; focus on the quantitative effects of white blood cells (WBCs) and red blood cells (RBCs) induced by applied radioactive dose of bone scan in NMI; bearing in our mind that the applied radioactive doses are: 740–1110, 111–740, 296–1110, 74–370 MBq for bone, renal, cardiac and thyroid scintigraphy respectively,17 and in general; the nuclear medicine dose ranged from 10 – 30 mCi.18,19
The innovativeness of this study derived from the unique in-vivo study revealing the effects of radioactive dose of bone scintigraphy in human blood components quantitatively with consideration to biographic data (BMI & gender). Also, the previous studies mostly concerned with the effect of radiation doses greater than that applied in diagnostic field (radiology & NM).
To quantify the induced effects in blood component; it is worth to highlight the normal count of blood components that may response to irradiation. The normal count of WBCs ranged from 4,000-10,000 counts\mm3 (lymphocyte (20-40%), monocyte (6-10%), neutrophils (40-60%), eosinophils (0.5-1) and basophils (0.5-1%)), RBCs are 4.8-5.8 million cells\mm3 in males and 4.2-5.2 million cells\mm3 in the females in addition to Plates counts from 100,000 to 400,000 platelets\mm3 (average 250,000). And the normal sizes of blood components RBCs = 6 to 8 micrometers, while the shape could be as round, biconcave and disc-shaped.20 All these characteristics of blood components could be influenced by ionizing irradiation and may show variable response as quantitative and morphological changes.
The estimation of Blood components in human body could be carried out by different means, such as 51Cr (Na2CrO4)-labeled red blood cells (RBCs) and 125I-human serum albumin to measure (RBCs) and plasma volume. 111In-oxine (oxyquinoline) and 99mTc /HMPAO (D, L-hexamethyl propylene amine oxime) Ceretec, exametazime are used to measure WBCs, while the induced morphological changes could be carried out from hematological and histological sections to reveal the induced changes relative to normal ones.
Since the Nuclear Medicine (NM) may accompanied with considerable side effects, particularly in the blood components, the aim of this research is to estimate the effects of radiation dose received by blood components (WBCs and RBCs) due to bone scintigraphy using 99mTc-MDP (Methylene Di-Phosphate).
The standard radioactivity dose for adults is based on the ideal standard weight of a patient, 70 kg,21 and the parameters such as pregnancy and sometimes renal function are considered as limiting factors to proceed the NM exam. Obtaining acceptable image quality for obese patient frequently requires higher doses than that needed for normal patient weight. However, the recommendation of International Commission on Radiation Protection limits the radioactive dose for imaging to be as low as possible, to guarantee the quality of image, obtaining proper diagnosis as well as to reduced signal-to-noise ratio and radiation scattering. Such recommendation is not concise with Positron Emission Tomography (PET) in which the imaging dose estimated according to patient weight to overcome poor image quality that caused by scatter, but should not exceed 925 MBq (25 mCi).22 As well as in general NM procedures; in which the activity calculated on the basis of patient weight and adjusted upward for heavier patients by using a fixed formula dose such as 11.47 MBq (0.31 mCi)/kg for 99mTc agents or 1.48 MBq (0.04 mCi)/kg for 201Tl or the general equation:
Another option to overcome this limitation and improve image quality is to prolong the acquisition time or use a multidetector system for higher statistical counts.21,23
The following study based on experimental sampling of cancer’s patient’s biography and blood sampling before and after bone scintigraphy. Then a quantitative, description and contextual approach has been applied for analyzed results using SPSS.
Sample & technique
The targeted sample consist of 150 cancer patients referred for bone scintigraphy for metastasis assessment at radiation & Isotopes Centre in Khartoum – Sudan during 2018. The patients have been informed for relative preparation (hydration, hair shaving and fasting) and on the due date they did laboratory test for WBCs and RBCs as routine blood checkup for cancer’s patients before radioactive dose administration for bone scintigraphy. Then the radioactive dose has been eluted from 99mTc generator based on its daily time activity formula ( , where I0 refers to initial activity and I refers to activity after elapsed time t and based on the disintegration constant λ) as total volume for all patients prepared for scan. Then the individual patient dose has been estimated based on body mass index (weight in Kg/height in m2) and further the relative amount has been mixed with MDP for each specific patient. The administrated dose composed of Technetium-99m (99mTc)/MDP; is given intravenously to each patient and the bone scan started after three hours for image acquisition as stated by Collier et al,.24 At this moment a 5 cc of blood sample has been withdrawn from each patient and sent to laboratory for further blood analysis and data collection such as: WBCs, RBCs using automated cell counter (Sysmex KX-21) together with the patient weight and height after taking patient informed consent.
Ethical point
The relative ethical approval obtained from the relevant institutions where the study takes place as well as the agreement of volunteered by signing the prepared form which contains the following statement: (the following data will be collected and used for the purpose of academic knowledge and publication in academic journals, and I (patient name: ………………., signature………….) agree and confess to participate as volunteer without any financial interest). The diabetic, hypertensive, malaria patients were excluded.
The following results present the common gender referred to bone scan in NM, correlation between WBC count and the age before/after 3 hours of radioactive dose injection, correlation between WBC count/cm3 and the BMI before and after bone scintigraphy, correlation between RBCs and the age groups in years before/after interval time of bone scintigraphy and the correlation between RBCs and the BMI before/after interval time (3-3.5 hours) of bone scintigraphy.
Figure 1 shows the Frequency percent of patients referred to bone scintigraphy, in which the female represented the predominant cases with a 70% compared with 30% male relative to total sample size (150 patients). The high frequency percent in female could be ascribed to the high incidence of breast cancer which referred to bone scan in addition to cervical carcinoma in contrast with male who presented for bone scan to assess the metastasis of nasopharyngeal and prostate cancers. Figure 2 shows the correlation between WBC count and the age before/after 3-3.5 hours of radioactive dose injection for bone scintigraphy. The data reveal that the WBC count increases eventually following the age group increment from 18 – 61 years old and then started to decreases but usually remain within the normal range (4000 – 7000/cm3) as agreed with the study done by Biino et al,.25 And as well same result had been presented by Valiathan and Asthana, et al,26 and Maulik et al,.27 Such normality is ascribed to normal human physiology (production and optosis). However the WCB count decreased by 3.8% (221.8) in average after the interval time of 3 hours of 99mTc administrated to patient, relative to initial count which is so significant at (P value = 0.00, t = 17.6), indicating that either the radiation destroying the WBC or could change their morphology causing the detector uncapable to distinguish and depict them or could be due to suppression of blood forming organs as stated by Sharma et al,.28

Figure 2 Shows the correlation between WBC count and the age before/after 3 hours of radioactive dose injection for bone scintigraphy.
Figure 3 shows the correlation between WBC count and the BMI before/after bone scintigraphy dose administrated to patient by three hours. It revealed that: the WBC increases as the BMI increases from ≤ 18.49 (underweight) up to 25–29.9 (overweight) then decreases among obese (30–39.9) and peaking among Morbidly obese (≥ 40 Kg) but remains in normal range. The activity dose (15±2.9 mCi) of bone scan reduces significantly the WBCs by 3.8% at P-value = 0.00 and P=value = 0.05) relative to age and BMI respectively. However, in both cases of aging and BMI variation, WBCs remains fluctuating within the normal ranges which are due to normal host physiology. But after the interval time of 99mTc dose administration, the WBCs count decreases by 3.8 % relative to initial count, which is so significant at (P-value = 0.05). The response of WBC to irradiation could be due to large target volume they possess for radiation photos and considerable radio-sensitivity.29 Also, the BMI together with the massive umber of RBCs/mm3, their small size, frequent rapid production and the radio-resistivity of RBCs could play a major role in the significance effect in WBCs and RBCs. Such role notable significant effects in WBCs compared with RBCs as obvious variation in t-test significances (P-value 0.00 for WBCs versus age and P-value 0.05 for WBCs versus BMI).
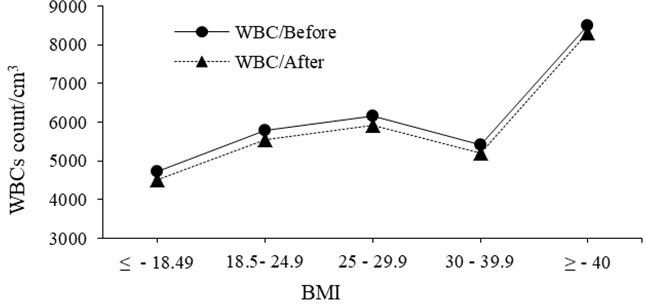
Figure 3 Shows the correlation between WBC count/cm3 and the BMI before and after bone scintigraphy dose injection (≤ 18.49 for underweight, 18.5 – 24.9 for normal, 25 – 29.9 for overweight, 30 – 39.9 for obese, and ≥ 40 Kg for Morbidly obese).
Figure 4 shows the correlation between RBCs and the age before/after interval time of bone scintigraphy. It shows that RBCs count has increases from 18 to 50 years old then decreases following ageing but remains within the normal amount along the age of man increment with an average of (4560800) cells/mm3 in contrast with the normal range 4.8 × 106 – 5.8 × 106 cells/mm3 or 6.46 × 103 / mm3 to 7.93 × 103 / mm3 for male and 4.2 × 106 to 5.2 × 106 cells/mm3 or 6.56 × 103 /mm3 to 7.33 × 103 / mm3) for females.27 However, after the interval time of bone scintigraphy the RBCs reduced by an average of 3.6% (165600 cells/mm3) relative to initial count and at an average dose of (15±2.9 mCi) as the t-test showed insignificant (P value = 0.31) reducing impact of radiation dose in the number of RBCs which is ascribed to damage of cellular membrane of RBCs.30 Comparing the impact of radiation dose in WBCs and RBCs, the impact was so insignificant in case of RBCs as it could be ascribed to small size and massive number of RBCs per mm3 compared with WBCs count. And the general reduction in WBCs and RBCs could be due to: either destruction of mature circulating cells, or failure of instrument to detect the blood corpuscles (WBCs & RBCs deformity) or cessation of WBCs and RBCs production which is occurs at high exposure doses.28,31
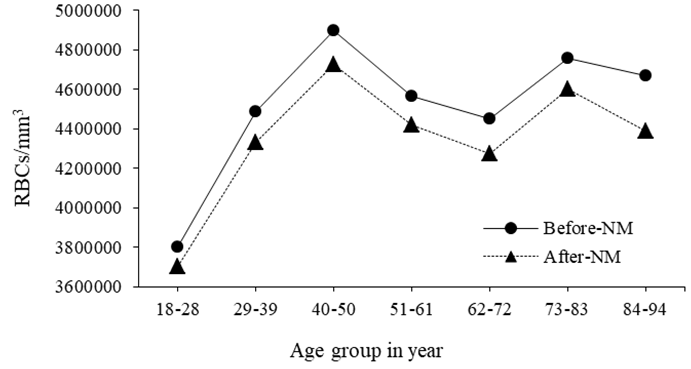
Figure 4 Shows the correlation between RBCs and the age groups in years before/after interval time of bone scintigraphy.
Figure 5 shows the correlation between RBCs and the BMI before/after interval time (3-3.5 hours) of bone scintigraphy. The data shows that: RBCs count has been increases as the BMI increases form underweight (≤ 18.49) up to normal BMI (18.5 – 24.9) then persist semi constant in normal range among overweight (25 – 29.9), Obese (30 – 39.9) and morbidly obese (≥ 40 Kg). However, the average radioactive dose of bone scan (15±2.9 mCi) reduces the RBCs insignificantly (P value = 0.32) by 3.6% relative to initial count. The massive umber of RBCs/mm3, their small size, frequent rapid production and the radio-resistivity of RBCs could enhance the negative effects of radiation.
The radioactive dose of Bone scan (99mTc/MDP) in Nuclear Medicine field; accompanied by adverse effects as reduction in WBCs and RBCs at doses of 15±2.9 mCi with regard to age and BMI, hence the count of WBCs and RBCs are necessary to be assessed before imaging acquisition started and the estimated radioactive dose better reduced as less as the applied formula gave; unless the scan have to be postponed till an ideal count of WBCs and RBCs maintain and the image quality could be achieved by increasing image acquisition time or using SPECT.
None.
The researcher would like to acknowledge the department of NM at radiation and Isotopes Center of Khartoum for their sincere assistance and encouragement during the preparation and implementing this work.
The authors declare that there was no conflict of interest regarding this manuscript.

©2020 Sakin, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.