MOJ
eISSN: 2379-6383


Case Report Volume 13 Issue 2
JEFE De Unidad Hematología & Hemostasia
Correspondence: Arana Matías, JEFE De Unidad Hematología & Hemostasia, Argentina, Tel +5491158897119
Received: April 25, 2024 | Published: July 4, 2024
Citation: Arana M, Cafruni G, Otero B, et al. Anemia predictive indicator (API). how can we predict in three days the anemia that will develop in three months? case report. MOJ Public Health. 2024;13(2):117-120. DOI: 10.15406/mojph.2024.13.00449
The Anemia Predictive Indicator (API) includes the analysis of the reticulocyte count, the reticulocyte hemoglobine content (HCr) and immature reticulocyte fraction (IRF). API reflects erythropoietic activity and allows immediate detection and correction of iron deficiency anemia, and the response can be observed on the second and third day after starting treatment. In this case report, a 79-year-old woman after a total knee replacement, API provided predictability at the onset of anemia, allowed the follow-up of anemia and its treatment, collaborated in clinical and medical decision, contributed to preventive actions and improve the patient's quality of life.
Iron deficiency anemia occurs in all ages without exception.1 It is a clinical condition that can affect mostly patients with chronic diseases and the elderly.2 Also includes pregnancy, renal disease, blood loss, important menstrual bleeding, inflammatory intestinal disease, bariatric surgery or extremely rare genetic disorders.3 Parameters traditionally used to study anemia include blood soluble iron, ferritin storage iron and transferrin saturation.4 However, these parameters are affected by certain conditions and are not easily assessed, especially during an acute phase reaction and in the presence of anemia of chronic disease.5 Factors such as inflammation, malignancy, liver disease and alcohol consumption increase those parameters independently of iron.6
The hemoglobin present in the reticulocytes (HCr) accurately reflects the quality of the red blood cells produced and their hemoglobinization7 due to iron metabolism of erythropoiesis in the previous 48-72 hours. The analysis of the reticulocyte count with the HCr and IRF indices allows immediate detection and correction of iron deficiency anemia, and the response can be observed on the second and third day after starting treatment. Changes in the iron status of erythropoiesis can thus be detected much earlier than by determining the hemoglobin content of mature erythrocytes only.8 In this way, it is possible to predict how these circulating red blood cells will be in the following three months.
Recent data from the World Health Organization (WHO) suggests that anemia affects about 800 million children and women with Iron Deficiency Anemia, being the most common.9 Reducing anemia is a key priority of the World Health Assembly Global Nutrition Targets 2025 and the Sustainable Development Goals.10
Blood samples were collected in BD Vacutainer EDTA K2 tubes. They were processed using the ADVIA 2120i Analyzer. The reagent used for Retic was AutoRETIC ADVIA 2120 (Oxazin 750).
A clinical and biochemical analysis of the results of the patient's hemogram and Retic was performed. It was done at baseline and days after knee replacement surgery. The biochemical parameters included: hematocrit, hemoglobin, mean corpuscular volume and the API parameters: absolute reticulocyte count; %Retic, HCr, fraction of mature reticulocytes Retic L (low absorbance), the immature fractions of Retic M (medium absorbance) and Retic H (high absorbance); and the count of immature reticulocyte fractions (IRF H+M) (Figure 1). The results for API parameters on the different control days were plotted and the API was used for clinical decision making and follow-up. The graphics show the mobility of different fractions according to the treatment and patient´s bone marrow erythropoiesis response. The hematologist physician takes decisions every control day described in the development.
Objetives
To use the API in a study case to:
Development
Hemoglobine (Hb) synthesis occurs only in the youngest reticulocytes in the bone marrow. Circulating reticulocytes can no longer synthesize Hb or further increase its concentration. Normal reticulocytes will mature one day in peripheral blood. In an acute bleeding episode, transient reticulocytes will rapidly leave the bone marrow and mature for 2, 3 or more days in peripheral blood. An increase in the immature reticulocyte fraction (IRF) means that the cells are produced rapidly, without indicating how efficient they are. The hemoglobin content in reticulocytes (HCr) could reflect the amount of hemoglobin and its capacity to transport oxygen, thus being a valuable tool for early diagnosis and management of anemias, mainly iron deficient with depletion of iron stores.
79-year-old woman needed total knee replacement. Baseline disease: Non-Hodgkin Lymphoma treated in 2017 and with routine bimonthly control due to chronic thrombocytopenia. Corticosteroids were indicated before surgery.
The pre-surgical examination was performed on 10/03/2022 and showed hematocrit (HCT) 46.8%, hemoglobin (HGB) 15 g/dl and mean corpuscular volume (MCV) 88.9 fl. On 10/05/2022 the patient went into surgery and left the hospital on 10/07/2022.
Control #1 (10/11/2022) a post-surgical control was performed with a Hemogram: it showed HCT: 25.9%, HGB: 8.4 g/dl (Table 1). The API was analyzed: absolute reticulocyte count: 158.5 x 10^9/l; Retic: 5.41% indicated activation of erythropoiesis. The HCr: 29.8pg indicated the existence of Iron available for erythropoiesis. A decrease in the fraction of mature reticulocytes Retic L (low absorbance) of 71.64% at the expense of an increase in the immature fractions of Retic M (medium absorbance) of 18.39% and Retic H (high absorbance) of 9.97%. The count of immature fractions (IRF H+M) was 28.36%. The anemia would develop if an iron treatment was not indicated.
Control # |
1 |
2 |
3 |
4 |
|
Date |
11/10/2022 |
14/10/2022 |
20/10/2022 |
26/10/2022 |
|
HTC % |
25,9 |
25,8 |
31 |
33,9 |
|
HGB g/dl |
8,4 |
8,6 |
9,8 |
10,7 |
|
RBC 10^12/l |
2,93 |
2,93 |
3,42 |
3,67 |
|
#RETIC 10^9/l |
158,5 |
265,9 |
344,5 |
173,9 |
|
RETIC % |
5,41 |
9,06 |
10,07 |
4,74 |
|
HCr pg |
29,8 |
33,6 |
30,8 |
30,1 |
|
RETIC L % |
71,64 |
62,42 |
70,14 |
74,59 |
|
RETIC M % |
18,39 |
22,35 |
19,43 |
17,23 |
|
RETIC H % |
9,97 |
15,23 |
10,43 |
8,18 |
|
IRF-H+M % |
28,36 |
37,58 |
29,86 |
25,41 |
Table 1 Patient´s Evolution. Control Days and parameters performed
On 10/12/2022, treatment with 40 mg Iron/Folic tablets orally for 45 days plus corticosteroids for thrombocytopenia was indicated. The Iron/Folic treatment was indicated because of the Hcr 29.8 pg. It was close to the lower value (28pg-36pg). Also an increase of % Retic, a decrease in the fraction of mature reticulocytes Retic L and an increase in the immature fractions of Retic M showed that the erythropoiesis was active and the anemia would not develop.
Control #2 (10/14/2022) a post-surgical control was performed with a hemogram: it showed a preserved HCT: 25.8%, HGB: 8.6 g/dl (Table 1). The API was analyzed: absolute reticulocyte count: 265.9 x 10^9/l; Retic: 9.06 % indicated high stimulation of erythropoiesis. The HCr: 33.6pg indicated effective increase of Iron available for erythropoiesis. A decrease in the fraction of mature reticulocytes Retic L (Graphic 3) of 62.42% was observed at the expense of a significant increase in the immature fractions of Retic M (Graphic 4) of 22.35% and Retic H (Graphic 5) of 15.23% (Graphic 1). The count of the immature fractions increased to 37.58% (Graphic 2). Anemia was controlled and treated in advance. Eritropoyetin was not indicated since API showed active Erythropoiesis.
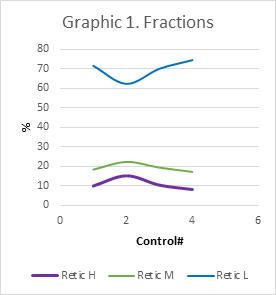
Graphic 1 Fraction A decrease in the fraction of mature reticulocytes Retic L of 62.42% was observed at the expense of a significant increase in the immature fractions of Retic M of 22.35% and Retic H of 15.23%.
Control #3 (10/20/2022) a post-surgical control was performed with a Hemogram: it showed a preserved HCT: 31%, HGB: 9.8 g/dl (Table 1). The API was analyzed: absolute reticulocyte count: 344.5 x 10^9/l (Graphic 6); Retic: 10.07 % indicated even stimulation of erythropoiesis by medication. The HCr: 30.8pg indicated a slight consumption of iron available for erythropoiesis. A slight increase in the fraction of mature reticulocytes Retic L of 70.4% was observed at the expense of a slight decrease in the immature fractions of Retic M of 19.43% and Retic H of 10.43%. The count of the immature fractions started to decrease 29.86%.
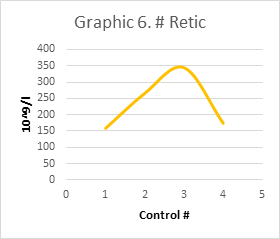
Graphic 6 #Retic An absolute reticulocyte count peak of: 344.5 x10^9/l was observed in day Control #3 after Iron/Folic treatment follow-up
Control #4 (10/26/2022) a post-surgical control was performed with a hemogram: it showed a preserved HCT: 33.9%, HGB: 10.7 g/dl (Table 1). The API was analyzed: absolute reticulocyte count: 173.9 x 10^9/l; Retic: 4.74 % indicated a stable and continuous erythropoiesis due to medication. The HCr: 30.1pg indicated slight stability of Iron available for erythropoiesis. A slight increase in the fraction of mature reticulocytes Retic L of 70.4% was observed at the expense of a slight decrease in the immature fractions of Retic M of 19.43% and Retic H of 10.43%. The count of the immature fractions was 29.86%.
Hb synthesis occurs only in the youngest reticulocytes (Retic) in the bone marrow. Circulating reticulocytes can no longer synthesize Hb or further increase its concentration. Normal Retic L reticulocytes (Graphic 3) will mature one day in peripheral blood. In an acute bleeding episode, transient reticulocytes Retic M and H, will rapidly leave the marrow and mature for 2, 3 or more days in peripheral blood (Graphic 4, 5). In the process of stimulation of erythropoiesis, an absolute increase in reticulocytes can be seen (Graphic 6). Simultaneously, a decrease in Retic L (Graphic 3) and an increase immature M and H fractions (Graphic 4, 5) are observed, thus the sum total of them IRF M+H (Graphic 2). An increase in the immature reticulocyte fraction (IRF) means that the cells are produced rapidly, without indicating how efficient they are. The hemoglobin content in reticulocytes (HCr) could reflect the amount of hemoglobin and its capacity to transport oxygen, hence being a valuable tool for early diagnosis and management of anemias, mainly iron deficient with depletion of iron stores.
An ineffective iron treatment would be diagnosed by the presence of microcytosis and hypochromia in the peripheral blood smear, with an expression only after three months. Other evidence of iron reserve depletion is given by classic markers such as: serum iron, transferrin, ferritin; but they can be altered by acute processes. The HCr is independent of these, presenting greater sensitivity and specificity11 and providing greater clinical usefulness at an early stage.
The API analyzes what happened in the bone marrow in the last 24 hours in a NON INVASIVE way, independently of the acute phase reactants. This will help medicine to avoid an Invasive Bone Marrow Iron study, contributing to patient´s quality life. API analyzes how efficiently bone marrow responds to correct anemia and how it compensates raw material production of transient reticulocytes with quality and quantity. Physicians take decisions to compensate with iron and/or EPO to correct anemia according to performance and mobility of IRF.
It diagnoses functional iron deficiency with greater sensitivity and specificity,11 in patients with HIV, cancer, sepsis, chronic diseases, acute bleeding. Most Anemia markers such us Ferritin and Iron don´t represent a diagnostic value during acute phases. It is the study of choice for follow-up in intensive therapies, post-surgery, post-dialysis, bone marrow transplantation, etc. It allows monitoring the Retic maturation analyzing the IRF mobility; Iron supplement analyzing HCr and EPO treatment analyzing how fast absolute Retic increase. Changes in the iron status of erythropoiesis can be detected much earlier than by determining the hemoglobin content of mature erythrocytes only. In this way, it is possible to predict how these circulating red blood cells will be in the following three months and treat the anemia in advance.
None.
The authors declare there is no conflict of interest.

©2024 Arana, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.