MOJ
eISSN: 2374-6939


Research Article Volume 15 Issue 3
1Postgraduate Program in Health Sciences and Rheumatology/ Ultrasonography Service, PUC-Campinas, Brazil
2Hospital Estadual Dr. Alberto Rassi, HGG, Goiania, Postgraduate Program in Health Sciences, Federal University of Goias (UFG), Brazil
3Faculty of Medicine, Federal University of Goias, Brazil
Correspondence: José Alexandre Mendonça, Postgraduate Program in Health Sciences, PUC-Campinas-SP-Brazil, Rua da Fazenda, 125,Vila Flora, Sumaré – SP - Brazil, Zip code: 13175- 665, Tel +55 019 8135-8322
Received: May 05, 2023 | Published: May 19, 2023
Citation: Mendonça JA, de Oliveira FMGP, da Silva NA. Ultrasonographic pulmonary evaluation in systemic sclerosis and control group with acr/eular criteria and high-resolution computed tomography. MOJ Orthop Rheumatol. 2023;15(3):94-97. DOI: 10.15406/mojor.2023.15.00625
The objectives of this study were to evaluate changes in the lung parenchyma in systemic sclerosis (SS) using ultrasonography; associate this findings with the clinical criteria and high-resolution computed tomography (HRCT). Methods: Thirty SS outpatients of two public hospitals in Brazil and ten healthy subjects underwent pulmonary ultrasound (US) examination, evaluating pulmonary parenchyma alterations in 14 intercostal spaces. The B-lines, structures indicative of pleural septal thickening, when present, were graded by a simplified score. HRCT of lung carried out previously were correlated with lung ultrasound. Results: Changes in pulmonary parenchyma seen by the US in such population were the B-lines, present in 96.67% of the patients. The classification of pulmonary US in this group was normal in 16.67%, mild in 30%, moderate in 36.67% and marked in 16.67% of the cases. Pulmonary US sensitivity in this study was 93% and specificity 77%. Patients with SS in the diffuse form had a higher number of B-lines than those in limited form. Correlation between pulmonary US and the number of B-lines was positive r=0.95; (p<0.001). HRCT scans with reports of pulmonary fibrosis presented more B-lines, r=0.54; (p=0.006). In the correlation between the semiquantitative US lung score and the ACR / EULAR Classification Criteria 2013, there was significant agreement (p = 0.010). Conclusion: The pulmonary parenchyma changes in SS identified through the US in this work were the B-lines. Pulmonary US with semiquantitative B-line score presented high sensitivity for interstitial lung disease (ILD) investigation. The manifestation of diffuse pulmonary parenchymal disease seen by the pulmonary US of this population was compatible with the results of previous studies.
Keywords: ultrasonography; ultrasound; systemic sclerosis; lung; interstitial lung disease, pulmonary fibrosis
There is an increasing technological advances of transducers and software and better image acquisition, with the evolution of portability and the technique of use, the ultrasonography becomes part of the arsenal of investigation and follow-up of several diseases, in the most diverse clinical areas.1,2 US has long been considered a poor-quality method to access the lung, but it has been observed in the last two decades that the presence of structures other than air in the lung opens an acoustic window and allows evaluation of pulmonary oedema and fibrosis, which can be directly seen and quantified.3,4 The role of pulmonary US in the evaluation of various pulmonary conditions has been previously reported.4–8 The main pulmonary manifestations in SS include interstitial lung disease (ILD) and pulmonary arterial hypertension (PAH), with or without pulmonary fibrosis.9 Pulmonary involvement occurs in approximately two-thirds of SS patients, and respiratory symptoms generally correlate with radiological evidence of pulmonary fibrosis and restrictive disease in lung function tests; being an important cause of morbidity and mortality.10,11 Some studies have been conducted to demonstrate the use of ultrasonography in the monitoring of pulmonary involvement in systemic sclerosis.3–5,12–20 B-lines, previously referred to as "ultrasonographic pulmonary comets" or "comet tails", are images that are detectable echographically, described in the evaluation of different forms of ILD; and consist of reflections of the US beam from the interlobular septum thickened by water or collagen, multiples, starting from the pulmonary surface. Interest in evaluating the presence of B-lines in patients with ILD is mainly to allow the early diagnosis of this pulmonary alteration and its follow-up, mainly when the scan is not available.3,6,12,14-16,21 This study is justified by the scarcity of publications regarding the use of pulmonary ultrasonography in our population with control group. There is no description in the literature, so far, of pulmonary US evaluation in systemic sclerosis in our country.
This is a cross-sectional, descriptive and control group study.
Population and sample
Thirty patients diagnosed with SS, with active records, attended at the rheumatology outpatient clinics of the General Hospital of Goiânia Dr. Alberto Rassi (HGG) and Clinical Hospital of the Federal University of Goiás (HC-UFG), both located in the city of Goiania - Goias (Brazil); and 10 healthy subjects were included in this study in a convenience sample, in 2016. Inclusion criteria were individuals aged 18 years or older who met the SS criteria of ACR / EULAR, 2013 (22) and able to understand and sign an informed consent form (ICF). Patients with overlapping syndromes, with associated infections or neoplasms, with any other lung disease other than ILD and with heart disease were excluded. The Ethics Committees of the two hospitals approved the study (2.007.032 – HGG / 2.098.991 – HC), and the ICF was obtained from all participants.
Data collect
After agreement and signing of the ICF, the participants performed the US exam on the day of their pre-scheduled consultations at the respective outpatient clinics or scheduled for the examination to be appropriate for them. Afterwards, data were collected from the medical records, related to the presentation of the disease, time of diagnosis, presence, and type of autoantibodies, clinical manifestations reported, and results of HRCT previously performed.
Pulmonary US evaluation
The pulmonary evaluation by the US method was performed in a physician's office, with a temperature stabilized at 23 °C, using a portable device of the ESAOTE brand (Genova - Italy), model MyLab25Gold equipped with a low-frequency convex probe (3.5 MHz), in B-mode.1 This transducer was chosen because it allows a good image of both superficial and deep structures in the lung.6 The patients were placed in dorsal decubitus for anterior chest evaluation and seated for posterior chest evaluation.
In the absence of consensus on how to perform focused lung ultrasonography6,8,23 and the expectation of making the examination feasible during outpatient visits, a simplified evaluation composed of 14 intercostal spaces was used, according to Table 1; demonstrated in Gutierrez et al (2011); because it is easily accessible and covers the main pulmonary segments involved with pulmonary interstitial fibrosis.
|
|
Anatomical lines |
Lung intercostal space |
|
Right and left anterior hemithorax |
Para-sternal |
2nd LIS |
|
|
Mid-clavicular |
4th LIS |
|
|
Anterior axillary |
4th LIS |
|
|
Mid-axillary |
4th LIS |
|
Right and left posterior hemithorax |
Paravertebral |
8th LIS |
|
|
Infra-scapular |
8th LIS |
|
|
Posterior axillary |
8th LIS |
Table 1 Anatomical sites by the simplified method of evaluation of B-lines by ultrasonography in SS patients
LIS, lung intercostal space.
Source: Modified from GUTIERREZ, M. et al. Arthritis research & therapy, v. 13, n. 4, p. R134, 2011.
The B-line was defined as a coherent, wedge-shaped or triangular echogenic signal with a narrow beginning near the edge of the image at the pleural line extending longitudinally by the pulmonary parenchyma to the edge of the image (Figure 1).3,21
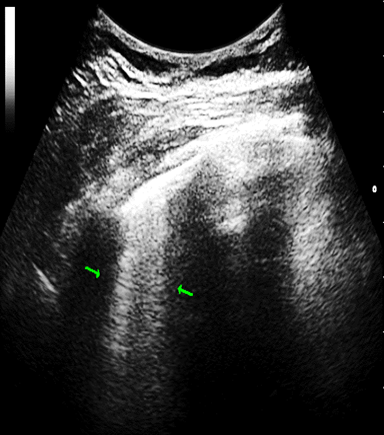
Figure 1 B-lines: vertical and perpendicular to the pleural echo, starting from it with comet tail. appearance. 2 B-lines (arows) in patient with systemic sclerosis (Author’s own imsage).
2 B-lines (arows) in patient with systemic sclerosis (Author’s own imsage).
The number of lines was counted in each intercostal space evaluated and subsequently added and graded according to the semiquantitative score, where it is considered normal <5 B-lines, mild from 6 to 15 B-lines, moderate from 16 to 30 B-lines and marked > 30 B-lines.21 The researcher who performed the US examinations of all the patients and controls presented experience and training in ultrasonography and had no previous information on the results of tomography or clinical data of the patients.
HRCT assessment
The HRCT were reviewed after the US, based on the patient's medical records. We evaluated those performed in the last 6 months prior to the implementation of the US; taking into consideration the presented reports, as to the presence or not of alterations of the pulmonary parenchyma, as (a) without abnormalities (normal), (b) pure ground glass opacity, and (c) pulmonary fibrosis (including reticular thickening, bronchiectasis and bronchioloectasias).14,21,23
Statistical analysis
Descriptive statistical analysis where continuous variables are expressed as mean and standard deviation, simple frequency and percentage. The categorical data are presented in absolute and relative frequency, counts and percentages. Inferential statistics were performed in the form of χ2 test (chi-square), Spearman's correlation coefficient or Mann-Whitney test as appropriate. For all analysed data, a significance level of 5% (p ≤0.05) was adopted. All statistical analyses were performed using Stata 13.0 software from 2013.
Demographic data for both groups are shown in Table 2. The feasibility of the US examination in this study was 100%, i.e., all patients and controls underwent the examination. The time to perform the pulmonary evaluation was on average 15 minutes. B-lines were the most observed structural changes in lung parenchyma, being present in the 29 (96,67%) patients evaluated and in 03 (30%) of the 10 healthy controls. The graduation of the semiquantitative US pulmonary score in the group of patients and in the control group are listed in Table 3. It is observed that 09 patients had a mild ultrasonographic score, where the number of lines ranged from 06 to 15 lines, and 04 of these patients had line number greater than 10. The positive predictive value (ppv) was 0.7 and the negative predictive value (npv) of 0.66 was evaluated for the presence of changes in the pulmonary US in patients with an IPD diagnosis. The sensitivity of the US lungs in the patients of this study was 93% and the specificity was 77%. The mean B-lines present in the pulmonary US of the 07 patients classified as ES diffuse cutaneous form was 26.9, while the mean of these lines in the 23 patients classified as ES cutaneous limited form was 15.8. Ten HRCTs were performed in a period equal to or less than 06 months after pulmonary US, and the correlation between the changes described in the HRCT and the semiquantitative score of the US positive but not significant (p = 0.3729) was recorded. The median number of B-lines was 24 in the group of patients with pulmonary alterations described in HRCT, while in those without HRCT changes the median was 6. In the correlation between the B-line quantification by the pulmonary US and the reports of the HRCT evaluated, those described as fibrosis presented a greater number of B-lines. In the association between the semiquantitative score of the pulmonary US and the presence of the ACR / EULAR Classification Criteria 2013, it is noted that those with higher scores in the classification criteria presents a higher degree of pulmonary parenchyma changes, with positive agreement (p = 0.010) (Figure 2).
|
|
SS patients (n = 30) |
Controls (n = 10) |
Female / Male (%) |
26 / 04 (86,7 / 13,3) |
09 / 01 (90 / 10) |
|
Age, mean (± SD) Min/Max (years) |
48,96 (13,4) 31 / 79 |
44,13 (14,85) 25 / 60 |
|
Classification Limited / Diffuse – n (%) |
23 (76,6) / 07 (23,3) |
__ |
|
Disease duration (years) Mean (± SD) Min/Max |
12,78 (9,59) 02 / 37 |
__ |
|
Smoking - n (%) Absent Current Ex-smoker |
26 (86,6) 01 (3,3) 03 (10) |
10 (100) 0 (0) 0 (0) |
|
Anti-Scl70 positive / investigated - n (%) |
11 / 18 (61,1) |
__ |
|
Anticentrômero positive / investigated - n (%) |
04 / 06 (66,7) |
__ |
Table 2 Demographic and epidemiological data of 30 patients diagnosed with SS and 10 healthy controls from two public hospitals in Goiânia - Goiás (Brazil)
SS, systemic sclerosis.
|
Semiquantitative score |
Patients n (%) |
Controls n % |
|
Normal (< 5 B-lines) |
05 (16,6) |
10 (100) |
|
Mild (from 6 to 15 B-lines) |
09 (30) |
0 |
|
Moderate (from 16 to 30 B-lines) |
11 (36,6) |
0 |
|
Marked (> 30 B-lines) |
05 (16,6) |
0 |
|
Total |
30 (100) |
10 (100) |
Table 3 Distribution of graduation of the semiquantitative US pulmonary score in the group of 30 patients diagnosed with systemic sclerosis and 10 healthy controls
This is the first study of pulmonary ultrasonography in SS performed in Brazil, particularly in the central-west region. The population of this study came from two tertiary public hospitals, which are a reference in the treatment of rheumatic diseases in our State and in several neighbouring states. This population corresponds to the epidemiological description of SS in the literature.9,11,14,21,23–25 US has been shown to be a very viable imaging method, as in this study, and it can be used in a wide range of medical areas, including bedside patients, with low-cost portability.6 Patients diagnosed with diffuse or limited SS evolving with interstitial lung disease had multiple B-lines on ultrasonography, corresponding to the findings of the study by Kielhauser et al.4 and Moazede-Fuerst et al.16 In the study by Lichtenstein et al.,7 the pulmonary US showed a sensitivity of 93% and specificity of 93% for the diagnosis of the interstitial syndrome in an intensive care unit. The high sensitivity of this method (93% in this study) demonstrates its importance for the evaluation of patients with SS that can progress with ILD, drawing attention to early pulmonary alterations and thus suggesting who and when to perform HRCT.14,16,20,21,23 As seen in Gargani et al.3 and Gigante et al.14 the B-lines observed here by pulmonary US have a correlation with the presence of interstitial lung disease evaluated in HRCT in SS patients. These results confirm that pulmonary US is a sensitive technique for the evaluation of this pulmonary manifestation in patients with SS. Ultrasonography can be used to follow up individuals with established disease and can become a viable method for the evaluation of SS patients with incipient pulmonary alterations.3,16 The pulmonary evaluation by US in patients with SS with pulmonary involvement associated with the simplified semiquantitative score of B-lines may benefit the diagnosis and follow-up of these patients as an additional, alternative or complementary method, with a significant correlation with HRCT.21,26 A systematic review and meta-analysis showed the AUC value of the SROC curve was 0.912 and 0.917 considering 9 studies, which indicated high sensitivity and low false positive rate for most of the included studies, high sensitivity was observed in our sample too.27 Our study corroborates the validity of this analysis, contributing to an increase in the number of patients evaluated by this score. It is shown that the ILD manifestations of patients with SS seen in the pulmonary US of this sample were compatible with the results and observations of previous studies.21,26,27 We are aware of limitations associated by the small number of rescued HRCTs does not allow a significant concordance or correlation with the severity of lung involvement in this study. Due to the lower incidence of this disease, the number of patients evaluated was limited. Most patients already had a well-established disease. In conclusion, our sample there was a favorable correlation with HRCT and B lines with an expressive sensitivity and specificity of this tool. It is sought to stimulate new centres to perform pulmonary US evaluation in SS or even to develop a multicentre study to standardize and validate this method for monitoring ILD in SS, to colaborate in the clinical practice of the reumatologist.
None.
The authors declare that they have no conflict of interests.
This project was not sponsored.

©2023 Mendonça, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.