MOJ
eISSN: 2374-6939


Research Article Volume 15 Issue 5
Department of Orthopedics, Hasharon Hospital, Rabin Medical Center, affiliated with Tel Aviv University School of Medicine, Israel
Correspondence: Dror Robinson, M.D., Ph.D. Department of Orthopedics, Hasharon Hospital, Rabin Medical Center, affiliated with Tel Aviv University School of Medicine, Petah Tikwa, Israel
Received: September 21, 2023 | Published: October 4, 2023
Citation: Robinson D, Gazit T,Yassin M. Treatment of low back pain with inhaled Cannabis, a 24 months follow-up study. MOJ Orthop Rheumatol. 2023;15(5):191-197. DOI: 10.15406/mojor.2023.15.00644
Chronic low back pain (LBP) is a common treatment-resistant musculoskeletal condition. The current study reports the results of therapy in 29 consecutive patients treated by cannabis inflorescences for a 24-months. All enrolled patients, failed at least on year of pharmaceuticals including anti-inflammatory agents, opiates and neurological medication as well as failed course of physiotherapy and had an advanced imaging study supporting an organic cause of LBP. Exclusion criteria were patients unable to consent (minors, prisoners, mentally incapacitated). The initial inhaled-MCT dosage was 20 grams per month of dried cannabis inflorescences consisting of 10:10 THC: CBD ratio (the allowed concentration range is ±4% thus concentrations ranged from 6-14%THC to 6-14%CBD concentration). MCT consisted of mixed cannabis strains. Females (5.89±2.0) required significantly lower dosages at 24 months than males (8.2±1.2). The primary endpoint was change in SF-12 PCS. This score significantly improved during 24 months of therapy (ANOVA F-value 46.3, p<0.001). The difference from baseline in SF-12 PCS become significant at 18 months and later. Secondary endpoints including VAS, ODI, SF-12 MCS demonstrated improvement during the MCT treatment period. In conclusion, MCT appears to be effective and safe treatment modality for chronic LBP with a high patient compliance and good safety profile.
Keywords: low back pain, medical cannabis therapy, Oswestry disability index, SF-12, PGIC
BMI, body mass index; BPI, brief pain inventory; CB, cannabinoid based; CBD, Cannabidiol; CI, confidence interval; CT, computed tomography; MCS, the mental component of the short-form 12 health survey (SF-12); MCT, medical cannabis therapy; MRI, magnetic resonance imaging; MOH, israel's ministry of health; PCS, the physical component of the short-form 12 health survey (SF-12); SF-12, patient reported outcome questionnaire; THC, tetrahydrocannabinol; VAS, visual analogue scale
Cannabinoid based (CB) treatments might change the course of chronic low back pain, a common condition that is often highly treatment-resistant and costly to society and insurers.1,2 Orthopedic related pain has been treated with CB products, with growing evidence of reduction of possible adverse side effects when compared to conventional treatments;3 However, CB treatments have not yet become an integral tool for treatment as part of the orthopedic conventional treatments’ toolbox due to cannabis being classified as a schedule one drug.4,5
CB treatments were found to be an effective form of pain control however the efficacy of the treatments varied by route of administration, with orally consumed having the most significant effect size, followed by oromucosal sprays and inhaled products.6 The differences in the pharmacokinetics were noted as potential causes for the differences in efficacy of those treatments.7
A significant majority of cannabinoid-based therapy protocols for alleviating pain use the dried flowers. The inflorescences are supposed to be vaporized by the patients, though many patients prefer to smoke the dried herb (despite the warnings of the perils of smoking). It was stated that smoking cannabis provides a rapid delivery, with THC reaching peak blood levels within 10 minutes with an approximated bioavailability of nearly 30%; yet major inter-subject differences were demonstrated despite using formulations with similar THC concentrations.6 These differences might be due to variability between smoking technique, puffs, inhalation duration and volume). Higher rates of dysphagia, sore throat, and headache were mentioned among the adverse effects recorded with regards to smoking cannabis.6
Low-back pain is a prime cause of disability, of diminished quality of life, and a leading cause for individuals to seek treatment; with total financial costs related to low-back pain exceeding $100 billion annually in the United States.3 Non opiate analgesics were noted as first line (i.e., nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen), to which often are added also muscle relaxants; and with regards to acute low-back pain systemic glucocorticoids are often prescribed; All those treatments bear the risks of long-term major side effects, which may exceed their benefits.8 Both acute and chronic musculoskeletal pain were noted as principal indications for opioid prescribed treatments, that were also stated to have a highly addictive potential and narrow toxic range (thereby pose as high-risk treatments), and as such contribute substantially to the continuity of the opioid epidemic.3,6,8,9 Cannabinoid-based treatments have a moderate-quality evidence of a small effect was present for duration of up to six months, with little evidence of severe adverse effects, and with potential to mild to moderate short-term adverse effects after use.6
Cannabidiol (CBD) was evaluated with regards to low back pain; Two recent reviews noted that patients reported of a beneficial effect on chronic pain after CBD treatments,10 and additionally noted the heterogenicity and shortage of studies and sufficient evidence. It was also noted that studies of high CBD treatments on pain were limited as neither dosing, dosing frequency, nor ideal dose combination was fully explored. Only a few studies have investigated the effects of medical cannabis therapy directly on chronic lower back pain11 or as an additive to gold-standard therapy.12
As most cannabis therapy protocols involve smoked dried-cannabis flowers and the most common orthopedic indication for cannabis therapy is chronic low back pain,13 this study evaluated the effectiveness of CB treatments in patients suffering from back pain for a minimal duration of 24 months receiving dried herb inhaled CB treatments.
This current study (CLN005) was a prospective observational open label study including 29 patient reported outcome questionnaires (ODI, VAS, BPI, SF-12) that have been treated between 2018-2021 in the Orthopedic clinic of Hasharon Hospital of Rabin Medical Center, Petah Tikwa, Israel. All 29 included patients had to comply with all the inclusion criteria and not to fulfill any of the exclusion criteria. Both primary and secondary end points which were based upon multiple-answer questionnaires filled by the patients during their follow-up visits to the clinic. This study was conducted in full compliance and in accordance with the ICH-GCP standards and with the ISO14155 declaration of Helsinki ethical standard requirements. This study was an investigator-sponsored study.
Study inclusion and Exclusion criteria
In accordance with local health regulations and requirements with regards to MCT, the inclusion criteria for this study were (1) Age above 18 years old; (2) Low back pain and sciatica diagnosed by a study independent orthopedic surgeon with documented sufficient treatment for at least 12 months; (3) Patients have been treated for at least 12 months with at least 3 categories of drugs, including at least one non-steroidal anti-inflammatory drug, one narcotic drug and one neurological drug (i.e. duloxetine, pregabalin, gabapentin, venlafaxine); (4) Failure of treatment in each of the 3 drug categories; (5) Failure of physiotherapy; and (6) Evidence on CT or MRI scan of disc for damage that may have caused radicular pain.
The exclusion criteria included (1) Evidence of prior psychotic reactions (all patients with known psychiatric conditions in the present or the past were evaluated by a psychiatrist to assess risk for drug abuse or psychotic reactions due to MCT, only patients cleared by such consultation were entered into MCT); (2) Lack of patient’s compliance to the above-mentioned drug treatments; (3) Use of antithrombotic drugs (i.e., coumadin, heparin) at treatment dosage.
Timepoints
Patients were evaluated at 0,1,3,6,12, and 24 months. During those clinic visits, pain was evaluated by the VAS scale answering the following question: “during the last week how intense was your average pain?”,14 quality of life by the SF-12 version 1 questionnaire15 and back related disability by the ODI.16 Additionally, the patients’ global impression of change was evaluated at 6 months and onward. BPI (brief pain inventory) was filled by patients in-order to consider dosage change. Adverse events evaluation was assessed at each visit according to a list of common adverse events with the possibility of reporting out-of-list adverse reactions by the patient.
Treatment protocol
All patients had received the same MCT treatment protocol. The initial inhaled-MCT dosage was 20 grams per month of dried cannabis inflorescences consisting of 10:10 THC:CBD ratio (the allowed concentration range is ±4% thus concentrations ranged from 6-14%THC to 6-14%CBD concentration). MCT consisted of mixed cannabis strains.
Criteria for dosage adjustment
Patients filled a daily consumption diary. Required dosage was calculated based on a presumed cigarette weight of 0.5 gram. Two cigarettes per day, require 30 grams per month. Concentration increase was based on results of BPI pain intensity score. A decrease of less than 3 grades in pain intensity grade or a BPI pain intensity sub-scale of over 4 was an indication to consider concentration increase.
Dosing adjustment schedule
Dose increase was considered after a minimal period of three months. Dosage modification was based upon the patients’ individual consumption records analysis. The monthly MCT dosage was increased to 30 grams monthly at 15:3 THC:CBD ratio with 11-19% THC to 0-7% CBD. Another potential dose increase was at six months. In subjects satisfying the dose increase criteria, the dosage was increased to 30 grams of THC20:CBD4 ratio with 16-24%THC and 0-8%CBD. Dosage increase was considered every six months thereafter according to the same criteria. A maximal dose of 60 grams of 16-24%THC and 0-8%CBD was allowed.
Recommended MCT daily consumption
It was recommended to start treatment with two puffs in the evening, and to add 2 more puffs during the following day, with dose escalation up to 6 times a day within three months.
Questionnaires and Endpoints
The primary endpoint was defined as a change in the PCS-subscale of the SF-12 (version 1).15 The secondary endpoints were adverse effects, dosage adjustments, changes in the PROMs. Questionnaire scoring was done as follows:
Statistical analysis
Statistical analysis was performed using Analyse-it for Microsoft Excel 5.90, 2022). Analysis of variance was used to assess the pain and well-being parameters according to questionnaires. Results are reported as mean ± standard deviation.
As the questionnaires were repeatedly administered over time control of false-discovery-rate (FDR) is needed. Essentially this is a method of assessing the rate of type I errors when a null hypothesis is tested using multiple comparisons. FDR is the expected proportion of wrong rejection of the null hypothesis. The equation is: FDR equals the expected ratio of the number of false positive classifications to the total number of null hypothesis rejections. The latter includes both false negative and false positive rejections.
Two different methods were used to control type I error:
The Benjamini-Hochberg correction (BHC) method is used to control FDR.22. The Benjamini–Hochberg method controls the False Discovery Rate (FDR) using sequential modified Bonferroni correction for multiple hypothesis testing. Another method to control the FDR is the Tukey-Kramer all-pairs comparison.23. It was performed on the continuous variables analyzed (SF-12, VAS, ODI) as well as the ordinal variable analyzed (PGIC). The all-pair comparison method controls for type I error and allows distinction of pairs of time-points in which the mean difference is significant.23,24
Ordinal variable (dose) was analyzed using Kruskal-Wallis test.
Patient compliance with the therapy was excellent. All patients continued the therapy for the 24 months follow-up period. Cohort demographics are reported in Table 1.
Parameter |
Mean±Standard deviation |
Range |
Age |
46.9±20.1 |
18-90 years |
Gender |
20 males; 9 females |
N.A. |
Weight |
85±19.4 |
45-115 kilograms |
BMI |
27.9±5.3 |
16-38 kilograms/meter2 |
Table 1 Cohort’s demographics
Dosing of MCT
Subjects required increased dosing over the course of two years. The dosing of subjects is described in Table 2. Maximal dosage allowed of 60 grams was reached in 3 patients. Females (5.89±2.0) required significantly lower dosages at 24 months than males (8.2±1.2) (Figure 1). Weight did not significantly correlate with dosage (Pearson’s r correlation coefficient 0.36, confidence interval -0.008 to 0.641). Age did not correlate to dosage at 24 months (Pearson’s r correlation coefficient 0.001, confidence interval -0.368 to 0.365.
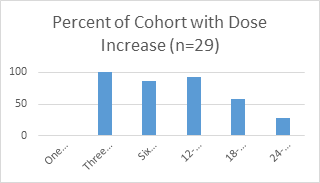
Per protocol dose increase was not allowed at one month.
The therapeutic response (ANOVA) with regards to pain intensity as assessed by VAS
Location |
|||||
ANNOVA |
|||||
Source |
SS |
DF |
MS |
F |
p-value |
TIME POINT |
658.6 |
4 |
164.6 |
76.08 |
<0.0001 |
Error |
303.0 |
140 |
2.2 |
||
Total |
961.6 |
144 |
6.7 |
||
Table 2 Dosage Changes over the 24 months follow-up period
H0=µ1 = µ2 = µ….
The mean of the populations are all equal
H1: µi ≠ µj for atleast one i,j
The mean of the populations are not all equal
Primary endpoint
The primary endpoint was change in SF-12 PCS. This score significantly improved during 24 months of therapy (ANOVA F-value 46.3, p<0.001). The difference from baseline in SF-12 PCS become significant at 18 months and later (Figure 2). The improvement continued at 24 months as compared to 18 months (mean difference 8.9, CI 1.7-16, p<0.0053). The Benjamini–Hochberg correction (BHC) indicated that the difference from baseline in PCS was significant at the 18 and 24 months timepoints (p< 0.000003, and p< 0.00000006, but not in earlier timepoints as compared to baseline. The difference between 18-month timepoint and the 24-month timepoint was significant (BHC, p< 0.0004). This finding indicates that the improvement related to MCT has not yet reached a plateau phase at 2-years.
Secondary endpoints
VAS
The therapeutic response (VAS as assessed using ANOVA) with regards to pain intensity was highly significant (Figure 3). VAS averaged 83±15.3 at baseline, and decreased to 35.3±19.3 (ANOVA, F-value 24.6, p<0.001). The BHC indicated that the difference from baseline in VAS was significant at the one month and later timepoints (p< 0.001) as compared with baseline VAS results. The improvement in VAS reached a plateau at 18-month, and the difference in VAS between 18-month and 24-month was not significant (BHP, p>0.06).
SF-12 MCS
SF-12 MCS improved significantly over the 24 months period (ANOVA, F-value 7.14, p<0.001)(Figure 4). The improvement became significant at the 3-months timepoint (Tukey-Kramer all-pair comparison mean difference 8.1, confidence interval 0.9-16.3, p<0.02). BHC indicated that the difference between baseline timepoint and 3-month timepoint was not significant (p>0.07) but was significant between 6-month and baseline (BHC, p<0.009).
PGIC
A responder analysis using PGIC (Patient’s Global Impression of Change) demonstrated that the patients have reported of general improvement in their condition, which was found statistically significant compared with the pre-MCT state from 6-months onward (Figure 5). The PGIC was skewed right at the 0 timepoint (Figure 6), and skewed left at other timepoints. This indicates that most patients had low-grade responses evaluating prior (non-MCT) therapy (Timepoint 0) and high-grade responses evaluating MCT (at later Timepoints). There was a gradual improvement in patients’ assessment of the therapy effect but it reached statistical significance only comparing the 6-months with the 24-months timepoint (Tukey-Kramer all pairs comparisons, 6 month - 24 month comparison, mean difference 0.8 grade, (CI 0.1-1.5) p<0.029).

Figure 5 PGIC change Comparing Baseline (results of prior therapy) with MCT from 6 Months to 24 Months.
PGIC was significantly different between the 6-month timepoint and the 18-month timepoint (HCS, p<0.02) and compared with the 24-month timepoint (HCS, p<0.007) but was not significant comparing 6-month timepoint and the 12-month timepoint (HCS, p>0.2). Patients’ assessment of the treatment was performed at baseline (evaluation of prior treatments including pharmaceuticals, surgery and physiotherapy), and at each consecutive timepoint from the 6 months timepoint and onward. Earlier timepoints were not assessed as the therapy protocol was being established and the patient needs to adjust to the therapy (dosage and consumption wise).
At 6 months the average PGIC score given by the patients was 5.6±1.2; when grade 5 represents slight improvement but not significant and grade 6 a marked improvement. At 12 months the average score was 5.9±1.1, and at 24 months the PGIC averaged 6.3±0.9 with grade 7 represents a remarkable improvement of the pain when compared to before starting MCT.
ODI
ODI improved during the MCT follow-up period (Figure 7). The improvement was gradual during the first year and was not statistically significant during the first year (Tukey-Kramer, mean difference 9.2, confidence interval -3.4 to 21.9, p>0.3). The difference in ODI was significant as compared to baseline, 1-month, 3-month, 6-month and 12 month, at 18-month timepoint (Tukey-Kramer, mean difference 24.1, confidence interval 11.4 to 36.7, p<0.001) and the 24 month timepoint (Tukey-Kramer, mean difference 27.1, confidence interval 14.4 to 39.7, p<0.001). Similar statistical significance was reached using the BHC method (baseline to 18-month BHC, p<0.0001 and baseline to 24-month BHC, p<0.0001). The differences between baseline and earlier timepoints than 18-month, was not significant (BHC, p>0.06).
The current study indicates that inhaled MCT retains good patient compliance during the 24-months follow-up duration. All the 29 patients have remained in the study for 24 months. Our findings also show a reduction in pain as measured by VAS as early as 6-months after initiation of MCT. This timepoint coincides with the change in some patients to 20% THC MCT which is more likely to allow pain relief as compared with lower concentrations of THC.7,25 The continuing improvement in pain scores indicates the superior analgetic-effect of inhaled T20 at least in cases of low back pain. Improvement in the study’s primary outcome measure (SF-12 PCS) was also significant when T20 inhaled MCT was used by the patients but not with lower concentrations of THC. SF-12 MCS improved earlier than the SF-12 PCS, with significant improvement already at the 3-months timepoint. At that timepoint, relatively low THC concentrations were used. The earlier mental improvement observed, appears to suggest that mental positive effects of inhaled MCT require lower concentration of THC than the physical effect in patients with low back pain.
A recent survey in North America of adults who legally self-administer cannabis for chronic pain (n=1087), of which 58% reported using to ameliorate back pain, had stated that 36.1% used inhalation-therapy, 45.1% used both inhalation and non-inhalation, and 18.8% used non-inhalation therapy;26 while the reported data from Israel of 110,971 patients showed that 89.7% of the permits were for dried inflorescence meant for inhaled therapy, and 10.1% were for extracts, and 56.6% were prescribed cannabis to treat chronic neuropathic non cancer pain.27 The difference in different localities regarding the routes of administration, may arise from availability of a wider range of cannabinoid-based products in different locales. Some such products have high THC content even up to 99.9% THC content,28 such as edibles which outlawed in Israel due to safety concerns. Inhaled cannabis leads to higher plasma-levels on THC with a short latency time from inhalation to maximal plasma concentration, in contrast, edibles consumption leads to longer sustained THC plasma levels.29 The most common method of consumption, and possibly the most efficacious26,30 is a combination of inhalation and non-inhalation products. Cannabis-usage preference appears to be gender related. Females and elderly males appear to prefer the non-inhaled methods of consumption.26,30 A recent meta-analysis appears to suggest that extracts (particularly high-CBD) are of minimal benefit in chronic non-cancer pain.25
The results demonstrate that there is a relatively fast response of pain as measured by VAS (significant change within 6 months of beginning of the therapy) but relatively prolonged response time with regards to physical function (SF-12 PCS and ODI) taking more than one year. At the same time patients’ global impression of change is positive at the earliest timepoint assessed (six-months). The positive patient global impression of change hints that the effect of cannabis inhalation might be related to other dimensions except pain and physical function. A support for the theory is the relatively fast mental improvement (SF-12 MCS) as compared to physical improvement (ODI, SF-12 PCS) observed in this study. The current study appears to indicate as well that relief of disability in chronic LBP requires high THC concentration in inhaled products as the improvement in ODI coincided with the period where most patients were treated by 20% THC inahalation therapy.
Nonetheless, these findings are meaningful with regards to MCT’s potential to reduce prescribed opioid treatments;26 as was also demonstrated by a long-term observational study,31 that reported that 50.8% of 61 patients suffering from low back pain discontinued opioids usage after receiving cannabis therapy, and out of the remaining 29 patients 31% were noted to have reduced their opioid usage; Moreover, it suggested that some patients may had been able to stop opioid usage if higher doses of cannabinoids were titrated until achieving the desired effect.31
The limitations of the current study are the limited number of patients included in the study and the observational non-randomized study design. The advantages of the current study are the multi-dimensional assessment of the effects of MCT on an homogenous cohort with a single musculoskeletal diagnosis of chronic low back pain. The study indicates that MCT is a successful therapeutic modality in chronic LBP, inducing pain improvement, increased physical function and high patient compliance.
None.
The authors report no conflict of interest.

©2023 Robinson, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.