MOJ
eISSN: 2374-6939


Literature Review Volume 15 Issue 6
1Department of Physical Medicine and Rehabilitation, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan
2Department of Physical Medicine and Rehabilitation, Tri-Service General Hospital, School of Medicine, National Defense Medical Center, Taipei, Taiwan
Correspondence: Shin-Tsu Chang, Department of Physical Medicine and Rehabilitation, Tri-Service General Hospital, School of Medicine, National Defense Medical Center, Taipei, Taiwan
Received: December 06, 2023 | Published: December 22, 2023
Citation: Shin-Tsu C. The role of quantitative sacroiliac scintigraphy in clinical relevance: a literature review. MOJ Orthop Rheumatol. 2023;15(6):233-237. DOI: 10.15406/mojor.2023.15.00652
Nuclear skeletal scintigraphy affords a highly sensitive functional method in order to identify systemic skeletoarticular disorders or injuries. Bone scan with quantitative sacroiliac scintigraphy (QSS) is a practical indicator for discovering inflammation/stress of sacroiliac (SI) joints. Sacroiliitis takes place in many situations, for instance, spondyloarthritis, osteitis condendsans ilii, post-streptococcal reactive arthritis, and lower extremity periostitis. QSS exerts a superior tool in scintigraphy rehabilitation and raises alertness for physicians paying more attention to the following steps for exact diagnosis.
Keywords: skeletal scintigraphy, osteitis condensans ilii, Streptolysin, periostitis, post-streptococcal reactive arthritis, scintigraphic rehabilitation
Skeletal nuclear scintigraphy affords a highly sensitive functional illustration for whole body in varying bony diseases.1,2 The technetium 99m-methylene diphosphonate (Tc-99m MDP) bone scan has been reported more of use than plain films in the earlier recognition of SpA.3 Tc-99m MDP, which is engulfed by skeleton cells. SPECT alone or combined with CT as hybrid imaging can identify the precise spot of pain in diverse disorder,4,5 the sternoclavicular joint inflammation.6 Quantitative SI scintigraphy (QSS) chose Tc-99m-MDP as the radiological compound.7 A recent article reported the worth of QSS in serving the differential diagnoses of backache-associated disorders, for instance, occult fracture,8 chiefly in patients with SI joint pain due to a traffic accident.
Nuclear medicine bone scan with QSS has been reported a valuable indicator for inflammation of SI joint in the last 60 years.9 QSS is performed by intravenous injection of 750 MBq Tc-99m MDP, and planar imaging of the spine/SI joints reaches in the antero-posterior projections 3 hours later. Based on the region-of-interest method, a SI joint-to-sacrum ratio (SI/S ratio or SI ratio), is calculated quantitatively by device computer.10 The ratio for the upper, middle, and lower thirds of both joints is recorded separately. Clinically, the inflammation is deemed as negative if the SI/S ratio is <1.3, or equivocal if around 1.4-1.5, or positive if >1.5.11
QSS perceiving and specifying sacroiliitis
Sacroiliitis is an inflammation of the SI joint of rheumatologic diseases or abnormal shearing force (stress), for instance, postpartum females, leisure runners, military soldiers, and ice hockey elites.12–17 Clinical expressions of sacroiliitis are lower back pain, and many SI- associated disorders.18
Buell et al.19 firstly reported elevated SI ratios in subjects with SI disorders. In 1998, Kaçar et al.20 calculated the SI ratios as average of <1.3200 for the healthy and <1.3812 for patients with late arthritis, while even elevated at 1.5200–2.0900 for patients with early arthritis. Tiwari et al.21 later gave an idea about quantifying ways of the SI joint index: rectangular ROI, irregular ROI, profile-integrated counts and profile peak counts. All of above four demonstrated akin to results. As a result, SI ratio is a excellent appraisal for vigilance of early but not late arthritis.20 Consideration of different tissue structures and kinetic biomechanics in the upper, middle, and lower three parts of the SI joints, SI ratios should be calculated separately in the three thirds.22 With respect to QSS in healthy people, the first literature reported that the SI ratios go downhill little by little with age in women, and there are two plateaus in ages 21 to 40, and 41 to 70 years in men.23 The novel quantitative analysis of QSS has been confirmed superior than planar bone scan in active stage.24
QSS for spondyloarthropathy, including ankylosing spondylitis
In the 1980s, QSS was utilized as an essential index in early diagnosis of ankylosing spondylitis (AS).25–29 The SI ratio of 1.55 indicated that AS steps forward to last less than three years or bigger than three years if 1.40.30 One study of AS patients with 2-year follow-up brought to a close that the QSS of AS proposes the prospect for earlier disease identification in possible cases.31 Considering time and cost, QSS is fairly superior than MRI in identifying AS as well as sacroiliitis.32 The factor of highly sensitive C-reactive protein (hs-CRP) is a biomarker for varynig diseases, such as SpA. A study declared that the high hs-CRP in SpA acts an index of SI inflammation to imply a rheumatologic upshot to a flair-up, based on the fact of a significant difference in hs-CRP and QSS, especial in the middle third.33
The magnitude of utilizing CT along with QSS in the judgment of SI joints is that combination of CT (semi-quantitative analysis) and QSS (quantitative analysis) can raise the inimitable requirement of the diagnostic intensity for active phase of sacroiliitis.34 CT can identify sclerosis and syndesmophytes, which is useful in last stage of sacroiliitis.34,35 For exact diagnoses of SpA, QSS is only advocated while examined together with the CT scan.64 The combination of both QSS and MRI increased the diagnostic accuracy sacroiliitis with axial spondyloarthritis (SpA).36
One review article enrolled studies about AS populations with/without sacroiliitis, and concluded that the probability ratio of QSS for diagnosis of acute sacroiliitis is only 2.5-3.0, so QSS is limited for diagnostic ethics for AS, even QSS is almost 50% cost and generally more avfeasible than MRI.37
QSS for osteitis condensans Ilii
Osteitis condensans ilii (OCI) was reported first in 1926, which was also called as idiopathic pelvis sclerosis,38 or hyperostosis triangularis ilii.39 OCI often shows intermittent lower backache and associated pain on SI stress tests,40 affecting women less than the age of 40. Patients also havd SI joint tenderness with obvious lumbar lordosis.41 The features of X-ray films of OCI reveal sclerosis in triangular shape along the auricular part of the both ilia with preserved space of SI joint,42,43 mimic radiographic sacroiliitis.44 OCI can bring stress in the SI joints, resulting in piriformis muscle syndrome with further sciatica. The relationship between OCI and SI joint sacroiliitis (stress), and between piriformis muscle syndrome and sciatica, has been reported. Symptoms of OCI showed with assocaited sciatica can be effectively treated by simple injection SI joint, which use QSS as the first to detect SI joint stress prior and after the injection.45 OCI is intractable in patient with SARS-CoV-2 infection, and higher ratios of QSS persisted even after intervention of multiple rehablitation modalities (Figure 1).

Figure 1 Osteitis condensans ilii is intractable in this case with SARS-CoV-2 infection, and higher ratios of QSS persisted even after intervention of multiple rehablitation modalities before (1A) and after (1B).
QSS for Post-streptococcal reactive arthritis
The development of post-streptococcal reactive arthritis (PSRA) has been known to be a prior subclinical streptococcal infection. The titers of anti-streptolysin O (ASLO) play a role in diagnosis PSRA,22,46–48 early arthritis related to rheumatic fever,49–51 as well as movement disorder.52 A defined cut point is considered as negative if the titer ≤116 IU/mL, and positive if >116 IU/mL.
Patients with high ASLO levels have bigger sways on all postural parameters compared to those with low ASLO. Proprioceptive deficit in the SI joint contributes to postural impairments.53,54 A study presenting the association of the SI joint in the progress of PSRA showed a significant relationship disclosed between QSS and ASLO, with the raised ASLO titer per unit, SI ratio raised by 0.0008 units, and furthermore 0.0074 units for every extra year, indicating a well-built association between both in PSRA.22 This is the only study presenting the involvement of this specific joint in PSRA.22 A latest case with ASLO value 241.4 IU/mL also present the similar finding (Figure 2).
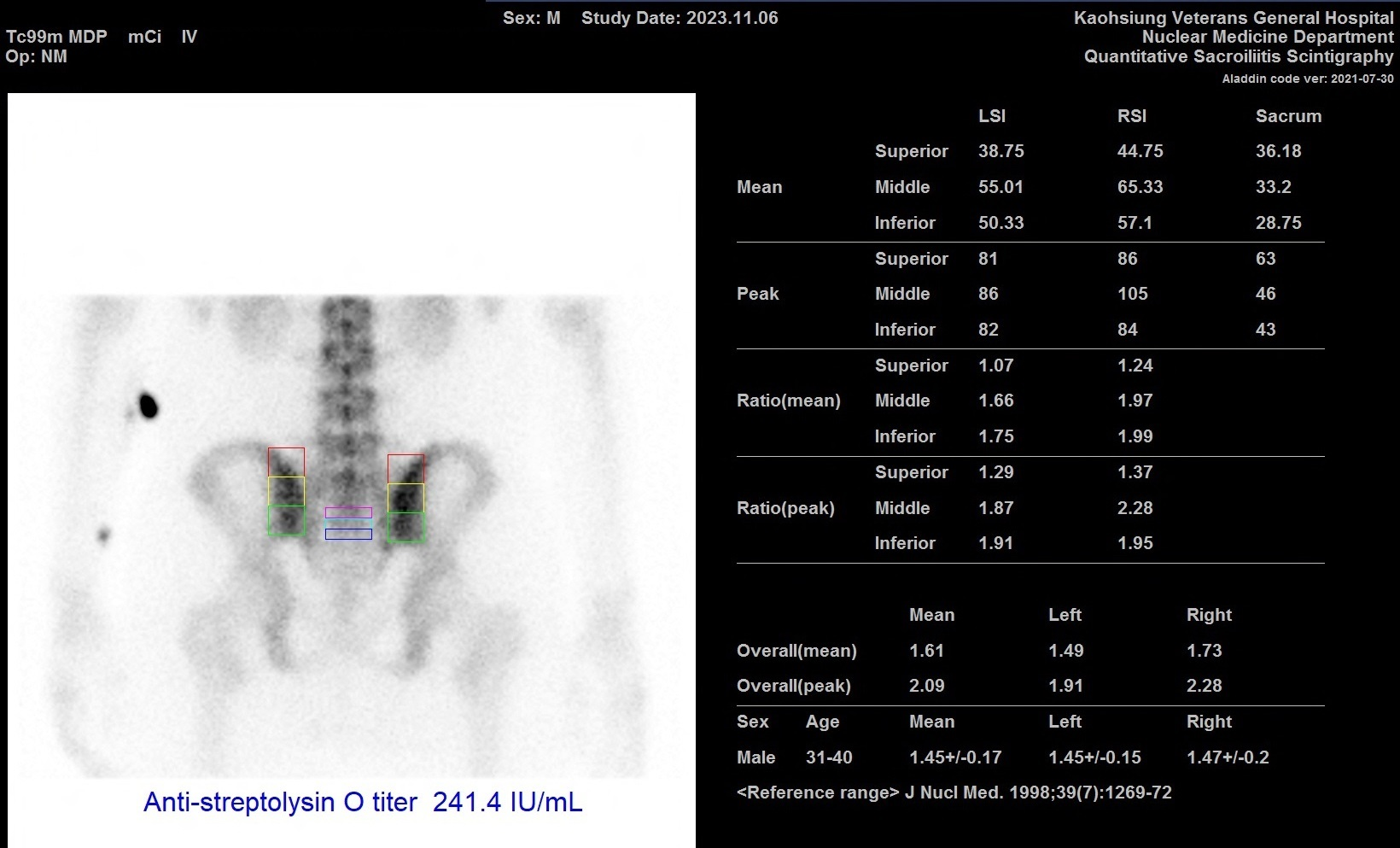
Figure 2 Post-streptococcal reactive arthritis can be diagnosed when measured ASLO value 241.4 IU/mL in this case with persisted higher ratios of QSS.
ASLO, anti-streptolysin O; QSS, quantitative sacroiliac scintigraphy
QSS for Periostitis-induced sacroiliac pain
Some body injuries, malpractice of exercise, and abnormal trunk posture will increase SI joint stress to develop high QSS, while pain-killerss and rehabilitation correcting abnormal posture are able to decrease SI ratios.55 Periostitis of lower limb (foot) is a common disorder in sports medicine, which can be treated well by a beneficial device, low-level laser therapy (LLLT), for improvement of proprioception disorder.56,57
Based on the truth that foot pain can bottom-up provoke SI joint stress, a noteworthy relationship was found among the middle and lower thirds of the joint, and QSS for the middle third on bilateral SI joints were significantly higher (0.06 units) compared to the lower third. Improvement of the foot periostitis can restore the abnormal QSS, which reflect to a conclusion that subjects with stress in SI after foot/ankle periostitis can be healed well by either LLLT or conventional therapies.55 Another study regarding medial tibial stress syndrome (MTSS) reported akin to results, which claimed that SI stress due to bottom-up processing of MTSS can be restored to normal after LLLT.58 Both MTSS and SI joint stress could be confirmed by QSS.58 The use of QSS is the first study of periostitis-induced sacroiliac pain.
Limitations of QSS
QSS has three imitations. The first is lower specificity in identifying chronic inflammation, the second is minute overlaps in SI indices in patients with/without sacroiliitis, and the third is lack of stratification of age and gender in order to discriminate the overlapping sites caused by sacroiliitis.
We revisited skeletal scintigraphy using QSS on patients with SI joint disorders, and recommand physicians paying more attention to the disease pathophysiology contributing sacroiliitis. QSS is ready to lend a hand in advisory evaluation of disease(s). Empirical practice of QSS showed that bone scan is an excellent technique for follow-up measurement in scintigraphic rehabilitation and raises awareness towards exact diagnosis.
None.
The authors declare no conflicts of interest. The article did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.

©2023 Shin-Tsu. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.