MOJ
eISSN: 2374-6939


Research Article Volume 10 Issue 6
Department of Orthopedics and Traumatology, Spine Surgery, Maggiore Hospital, Italy
Correspondence: Amendola L, Department of Orthopedics and Traumatology, Spine Surgery, Maggiore Hospital, 'C. A. Pizzardi', Largo Nigrisoli 1, 40100 Bologna, Italy
Received: February 24, 2018 | Published: December 27, 2018
Citation: Amendola L, Cauli V, Calabrò T, et al. The importance of early diagnosis and management in total knee arthroplasty infection. MOJ Orthop Rheumatol. 2018;10(6):399-411. DOI: 10.15406/mojor.2018.10.00457
Periprosthetic joint infection (PJI) is a devastating complication after total joint arthroplasty and is often associated with poor clinical outcomes. Diagnosis can often be challenging and is based on conventional and molecular strategies. Treatment is complex and depending on many variables: timing of infection, bacteriology, implant stability, host condition, soft tissue impairment. Hence orthopaedic surgeons, microbiologists and infectious-disease physicians should work in a multidisciplinary-team for an optimal treatment that includes different options: antibiotic-suppression, debridement, one- or two-stage prosthetic replacement and arthrodesis.
The authors emphasize the importance of timely and accurate diagnosis and management to prevent long and costly procedures in case of false–positive diagnosis and to prevent recurrent implant failure in false-negative cases.
Keywords: Total knee arthroplasty, infection, diagnosis, medical treatment, surgical treatment
The infection of a knee prosthesis is a serious complication often characterized by a complex and prolonged diagnostic procedure and the treatment of which, even if could gain a healing of the septic process, not always results in a satisfactory functional result.1 Difficulties in the treatment of a knee prosthetic infection deal with the presence of the prosthesis which acts as a foreign body, feeding the persistence of the infection itself2. Microorganisms usually considered as non-pathogenic or poorly resistant to common antibiotic therapy could determine the clinical conditions in which the healing can be achieved only with the removal of the prosthetic implant3. There are particular situations at the base of the onset and persistence of so-called "foreign body" infections: the host organism reacts against biomaterials filling them with a thin film made of proteinaceous material. Some of these proteins have receptors specific for Staphylococcus Aureus and Epidermidis, determining contact that at least initially, is reversible. This condition can become irreversible through the production of a gelatinous substance (exopolysaccharides) by certain bacterial species as coagulase-negative staphylococci (St. Epidermidis, Capitis, Haemolyticus), Enterobacteriaceae, Pseudomonacee, Aciteobacter (Figure 1). Formation of a matrix glycoprotein then acts, defined by Gristina and Costeron, as "glycocalyx".2 The action of the glycocalyx is manifold: it forms a barrier against antibiotics and inhibits macrophage phagocytosis.3
Three forms of PJI can be classified according to the route of infection: perioperative (inoculation of microorganism during or immediately after surgery), contiguous (from an adjacent focus of infection) and hematogenous (microbial spread through blood from a distant focus of infection). Another classification is based on the onset time of symptoms after implantation: acute (less than 1 month) and chronic (more than 1 month). Acute infections are generally perioperative forms and caused by highly virulent organism (St. aureus, Gram-negative bacilli, anaerobic or polymicrobial infection); chronic infections are predominantly hematogenous or perioperative forms and caused by low-virulence organism.4
Since knee prosthesis (-es) began to be used, the incidence of prosthetic infection after total knee arthroplasty (TKA) was 23% of cases.5 During Seventies, this percentage has been reduced by up to 5%,6–9 while recent data reported by the Swedish register of knee replacements, certify a rate between 1% -2% of cases.10 The high incidence of infections reported in older series related to a period before the modern antibiotic prophylaxis, which has been proved to be the most important factor to limit the infection. The frequent use in those years of hinged prostheses has contributed to maintain the high rate of infections (incidence infection rate between 11% and 16% with these implants, even with appropriate prophylaxis).9,11 To explain the vulnerability of knee joint to infection in addition to the intrinsic characteristics as the shallowness of the joint and absence of adequate muscle coverage,12 the literature reports various conditions related to the patient or surgery which are listed in Table 1.6,13–15
|
Patients-dependent factors |
Surgeons/operating room dependent factors |
|
Immunologics deseases · Reumatoid Arthritis · Diabetes mellitus
· Neoplastic deseases Obesity Concomitant Infections · Urinary track · Skin · Dental Previuosly infections · Septic arthritis · Osteomyelitis Smoking Previous surgery Skin (psoriasis, eczema, ulcers) Alcool abuse Malnutrition (serum transferrin <200 mg/dL, serum albumin <3.5 g/dL, total lymphocyte count <1500 cells/mm3)
AIDS/ HIV Older patients (>75 yr)
|
Surgical environment · Operating-room traffic · Airflow
Surgical team · Gloves · Dressing · Personalized protection suit
Intraoperative Surgical Factors · Prophylactic antibiotics · Skin preparation · Draping · Bleeding control · Antibiotic cement · Skin closure · Prolonged operative time (ie, > 2.5 h)
|
Table 1 Risk factors for infection of the total knee arthroplasty
Infection risk is very high in patients with compromised immune system, as has been clearly demonstrated in rheumatoid arthritis, in which this complication is 2.6 times higher than in patients with osteoarthritis.14 Diabetes raises the incidence of infection up to 3% to 7% of cases.16 In the preparation and evaluation of a patient for a programmed intervention of arthroplasty, careful research of any infectious foci at the level of' odontostomatological apparatus, the genito-urinary tract, and respiratory system must be performed on all patients, in order to treat them immediately before surgery, as a source of infection by hematogenous bacteremia. Finally, is uncertain the incidence of infection in the presence of psoriatic lesions; Stern et al.17 reported an incidence of infection of 17% in patients with psoriasis, but has not been confirmed by subsequent studies.
Clinical evaluation
The clinical scenario largely varies. In many acute infections, there is a sudden onset of one or more of the following symptoms: pain, which does not heal with rest and worsens with load-bearing, stiffness, local heat, swelling, and sometimes fever. This condition is often preceded by superficial necrosis and/or persistent discharge from the surgical scar as blood serum, serous and finally purulent. The presence of a sinus tract, finally, documents the subfascial extension of the infectious process. In contrast, chronic infections usually don’t have many of acute signs and symptoms; they have an indolent course characterized by persistent joint pain with or without early implant failure (within 2-3 years after implantation), which makes it more difficult to distinguish from aseptic loosening.18
Recently, it has been demonstrated that prosthetic loosening within 2 years of implantation is highly predictive of infection. From a clinical point of view, mechanical aseptic loosening may present with pain with joint motion and weight bearing, whereas joint pain from a chronic infection may also occur at rest.4
Imaging studies
In the early period standard X-rays are usually negative, while the presence of signs of radiolucency, osteolysis, or migration of the prosthesis are common in case of mechanical loosening.4,6,12,18,19 Scintigraphy is indicated in cases of a valid suspicion of infection;4,20 scintigraphy with labeled leukocytes is able to assess the existence of an inflammatory focus and to localize it by means of 99m Tc-labeled leukocytes that massively moves by chemotaxis and are found in large numbers in the inflammatory focus and, in doubtful cases, accuracy is enhanced by the use of radiocolloid.20
Laboratory studies
Laboratory testing for infection include nonspecific inflammatory markers that could be elevated in noninfectious diseases as well as in the early postoperative period. They include leukocytosis with significant increase in the percentage of neutrophils, increase in erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). In most cases, however, leukocytosis is uncommon and often not significant;5 also the ESR has a limited specificity in the study of a knee prosthesis sore.4,6,18,19 The CRP showed greater specificity as it tends to rise in the presence of infection while doesn't modified in the presence of a mechanical loosening of the prosthesis.6 In uncomplicated cases the CRP reaches maximum values at the 2nd-3rd day post-operatively and tends to be normal after 3 weeks, while in the presence of an infection process were consistently higher (Figure 2).4,6,12,18 Hence it is considered the cheapest test to rule out infection.
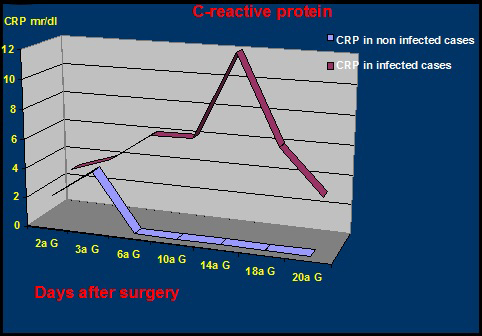
Figure 2 Comparison between CRP titer in infected and non-infected total knee arthroplasty (Courtesy of Prof. F. Ghisellini).
Recently, other biomarkers of inflammation including IL-6, TNF-alfa, and procalcitonin has been investigated as biomarkers of PJI. Serum IL-6 reaches the peak 2 days after uncomplicated arthroplasty and rapidly returns to normal value. On the basis of a recent meta-analysis, it has the highest accuracy for the diagnosis, followed by the PCR? The ESR rate and the white blood cell count. About the TNF-alfa value there is a scarce of information in the literature and about procalcitonin , several studies demonstrated that a cutoff level of 0,35 ng/ml revealed a sensitivity of 80% and specificity of 37%.5
Microbiology
The identification and study of bacterial sensitivity to antibiotics constitute an important element for prognosis and subsequent treatment. The collection of biological material to be sent to the laboratory should be aimed to the identification of aerobic and anaerobic bacteria, and fungi and mycobacteria in doubtful cases. Three different types of samples could be obtained preoperatively: blood cultures, fistula exudates and synovial fluid.
Blood cultures, despite are easily obtainable, have little use because have an extremely low sensitivity and specificity. Cultures from a fistula exudates are considered a useless tool because the most frequently isolated organism from these infections are common skin contaminant and can be isolated from the skin of the fistula, with scarce specificity.4
Aspiration of synovial fluid by fine needle aspiration has been questioned about the possibility of false negatives.6,18 Windsor and Insall19 consider this exam as fundamental for the diagnosis of infection in total knee arthroplasty. Duff et al reported data sensitivity, specificity and accuracy of 100% by needle aspiration before total knee revision surgery.21 In order to avoid false-negative fine-needle aspiration should be performed after discontinuation of antibiotic therapy at least 2-4 weeks before. If the aspirate fluid is negative but there remains a suspicion of infection, should be made at least two other attempts.21 In some cases where the diagnosis remains uncertain is recommended incision biopsy with multiple specimens from synovial and bone-prosthesis interface,6,22 because microorganism are embedded in biofilm and the synovial fluid could be sterile. In general, culture of fluid sample and soft tissue from around the implant for all revision arthroplasty is crucial (a minimum of 3 specimens should be sent to the laboratory), although in 7% to 11% of patients with prosthetic joint infection, cultures are negative. This can be explained with the capacity of microgranism to form a protective biofilm and to switch to a dormant metabolic form with small colony variants. Therefore a preoperative antibiotic treatment, reduces the sensitivity of microbiological culture of the tissue around the implant.23
Role of alfa-defensin
Unfortunately, there is no single test or finding that is 100% accurate for the diagnosis of PJI. About this, differents studies demonstrated the potenial clinical benefit of synovial fluid alfa-defensin levels. Defensins are a family of antimicrobial peptides that present a part of the innate immune system , whose role is to directly neutralize invading pathogens without the need of prior sensitization. Alfa-defensins are primarily expressed in polymorphonuclear cells (PMNs)and nongranulocytes cells including natural killer cells. In PMNs they play a role in the oxygen –dependent killing of phagocytized pathogens and showed broad-spectrum antimicrobial activities against gram-positive and gram-negative bacteria. So they have been identified as markers of microbial activity in the innate inflammatory response and have shown potential promise in improving the accuracy preoperatory and intraoperatory diagnosis in PJI. In addition, alfa-defensins have been shown to outperform others biomarkers investigated as leukocyte esterase test strip, as well as diagnose PJI correctly 99% of the time when combined with synovial fluid C-reactive protein (CRP). Bingham et al performed a retrospective review of 57 patients undergoing PJI workup and demonstrated alfa-defensin to have a sensitivity and specificity of 100% and 95% respectively, demonstrating good diagnostic accuracy of this test for first –stage or single –stage revisions. Moreover if this immunoassay is not concordant with the presumptive diagnosis, serious consideration should be given to the possibility that the presumptive diagnosis is incorrect, triggering further clinical evaluation. Future research should focus on improved bacterial identification techniques and on specific clinical scenario (for example immediate postoperative patients or immunocompromised).24–26
Sonification and molecular biology strategies
As the differential diagnosis between asepting loosening and septic failure can be very diffiult, because clinical features are ambiguous and laboratory – imaging tests have notable rates of false positive and false results, further efforts are being made to improve our diagnostic capabilities. Sonication is a method based on the use of ultrasound that disloge biofilms from the surface of implants . The material obtained is subsequently placed in culture for a week. This tecnique can increase the sensitivity of microbiological studies from 54,4% to 60,8 % for peri-prostethic tissue cultures and from 66,7% to 78,5% for fluid cultures. Puig-Verdie’ et al. found in their study the significantly higher sensitivity of implant sonification compared with peri-implant tissue culture for the diagnosis of infection in delayed, but not early, failure. Thus, it can be a valuable aid in the differential diagnosis of aseptic loosening and septic faiure of the implant, even if the cultures also obtained by sonification have limited sensitivity in patients receiving antibiotics.
This can explained because early failure are usually caused by culture rapidly –growing micro-organisms that may not have had enough time to produce biofilm. The absence of biofilm explains the limited use of sonification and the higher sensitivity of tissue culture. Even if sonication has proved superior to conventional methods there still remain some clinically infected cases with negative cultures. For this, reason the use of sonification of the implants is not fully recommended in all guidelines. However, most authors think that the use of sonification cannot substitute the culture of periprosthetic tissues or any samples, but it should be performed whenever possible, because implant processing add valuable data that can help to the management or interpretation of all microbiologic results. When sonication cannot be performed because of a lack of technology, vortexing of implant has been demonstrated to be a viable alternative. Despite studies showing poorer results of vortexing than sonication for chronic infections or in patients with previous antibiotic therapy, the results are better than with conventonal cultures and very interesting.
Esteban et al. In their study have evaluated if an incubation time of two weeks decreased the number of negative cultures when compared with conventional seven day incubation. They found that the use of prolonged incubation times did not increase the detection of infection and when mycobacterial or fungal infection is suspected , special tissue cultures should be performed. However there are organism that are difficult to detect even if this approach is used. In these cases, the use of molecular biology including 16s rDNA amplification and sequencing is needful.
PCR (polymerase chain reaction) is a technology that enzymatically amplifies deoxyribonucleic acid (DNA) by means of sequence-specific oligodeoxynucleotide primers, to detect bacterial DNA. It is a rapid techique that provides definitive results in half day (compared with one week for culture). Many studies have shown high sensitivity but have been limited by the number of potential false-positive results. With the persistence of bacterial DNA after cell death, any bacterial contamination, even in an antibiotic –cleared infection, could cause a positive result in an otherwise sterile sample. To overcome this aspect, Bergin et al. in their study, explored the utility of mRNA (messenger ribonucleic acid) with use of reverse transcription – quantitative polymerase chain reaction (RT-qPCR) in place of DNA. They found that this strategy had higher specificity but lower sensitivity, due to the naturally low number of mRNA transcripts in the bacteria present in a clinical sample and high rate of degradation of mRNA after cell death. In front of this limited aspects, they evaluated the use of rRNA(ribosomia ribonucleic acid) hypothesizing that it would have higher sensitivity compared with mRNA because it make up more than 95% of the total RNA content. Moreover rRNA is a molecule more robust and is able to overcome more easily the extraction process. Interestingly, there is a delay of approximately one week during which rRNA detection persist although the samples remain culture -negative. Thus, a negative rRNA RT-qPCR assay may be used for assessing infections that have been partially treated with antibiotics (as the results of culture are notoriously poor for these patients) and in case of two-stage treatment to determine the appropriate timing of reimplantation. An other equally important aspect is that rRNA own highly conserved regions, so all bacterial pathogens can be rapidly identified with the universal primers. Similar highly conserved regions in mRNA are infrequent, making universal bacterial detection more difficult with mRNA in comparison with rRNA. In conclusion, molecular diagnostic methods compared with intraoperative tissue culture, especially if combined with sonication, have a higher sensitivity, a faster turnaround time and are not influenced by previous antimicrobial therapy, even if they still lack a system for detetion of antimicrobial susceptibility.
Treatment options for managing an infected TKA can be generally categorized as prosthetic retaining, prosthetic exchange and salvage procedure. Belong to first category suppressive antibiotic therapy and debridement with irrigation, which may be performed arthroscopically or through an open arthrotomy. The second category consists on prosthetic removal and subsequent reimplantation. According with the timing of the reimplantation, the exchange procedure is differentiated in early exchange (or one-stage procedure) and delayed or two-stage procedure. In case of one-stage technique the surgeon remove all the implants and cement, and thoroughly debride all infected tissues, excide all sinus tracts if presents, and install new implants using an organism-specific antibiotic cement during the same surgical procedure. The two stage exchange consists on the same meticulous debridement procedure followed by the implantation of an antibiotic-loaded spacer. The spacer acts as local antibiotic-delivery system to maintain limb length and the anatomical relationship of the joint. The second definitive reimplantation procedure is delayed of six to eight weeks; during this time intravenous or oral antibiotic therapy is administered based on recommendations of the infectious disease. A close clinical evaluation of the patients as well as the monitoring of hematological tests (ESR, C-reactive protein) complete most protocols for delayed reimplantation. The optimal outcome of treatment of an infection at the site of a total knee arthroplasty is restoration of a painless, well functioning joint and eradication of infection. In some cases, the infection is eradicated but it is not possible to retain good joint function, in which case arthrodesis or amputation may be the end result, which is considered as salvage procedure.6,10,12,18,23–33
The choice of the best treatment is based on several factors including the characteristics of the patient, the type of germ involved, the duration of symptoms and the condition of bone and soft tissues. Recently Tsukayama et al.34 have proposed a clinical classification with the aim to help the surgeon to make a choice of the appropriate management. According to this classification there are only two circumstances when surgery is not needed. The first one is a very superficial suture infection that usually can be treated with oral antibiotics. The second circumstance in which surgery may not be indicated is when the patient has a positive intraoperative culture. In this setting, a culture of an intraoperative specimen that is obtained during a revision arthroplasty for presumed aseptic loosening is subsequently found to be positive, usually for a coagulase-negative staphylococcus. The main consideration is whether the positive culture represents contamination or infection. To avoid this dilemma, it is recommend to obtain multiple specimens at the time of revision surgery, even for presumed aseptic etiologies, and making the diagnosis of infection only if the same pathogen is isolated from more than one specimen. The validity to obtain multiple specimens has been demonstrated by Atkins et al.22 who performed a prospective trial in 334 patients: they observed that at least three positive specimens are necessary to confirm diagnosis of infection. Treatment with antibiotics alone, without further operation, is curative in 90% of these situations.
In all other instances of deep infection, surgical intervention is indicated to adequately debride infected tissue. The most important decision to consider in this scenario is whether to remove the prosthesis. An attempt to salvage the involved prosthesis is reasonable when the infection presents acutely. There are two types of acute infections: early postoperative infections (EPOI) and acute hematogenous infections.11 By definition, an EPOI occurs within one month after implantation of the joint prosthesis. An acute hematogenous infection represents hematogenous seeding of the joint from another primary site of infection. Both types of acute infection present with local inflammation of acute onset and with systemic toxicity, and in these cases operative debridement is mandatory.
Antibiotic suppressive therapy
Antibiotic treatment is rarely used alone, as is unlikely to cure deep infection about knee prosthesis.3,6,10,12,18,23,33–35 However, in extremely sick and frail patients who may not tolerate surgical treatment, antibiotic alone may be used to control infection and prevent systemic progression. For this reason antibiotic management is defined as a suppressive treatment. The aim of the treatment is in fact those to arrest the grow up of the microorganisms. Antibiotic treatment alone should be considered in particular cases, as this treatment will not eliminate deep infection and is commonly associated with very poor results (Table 2).
|
Author |
N° of cases |
N° of success |
|
Insall JN Instr Course Lect 1982 |
6 |
2 |
|
Woods GW Clin Orthop 1983 |
7 |
4 |
|
Grogan TJ JBJS 1986 |
3 |
1 |
|
Johnson DP JBJS 1986 |
25 |
2 |
|
Bengtson S Acta Orthop Scand 1991 |
225 |
44 |
|
Wilson MG JBJS 1990 |
8 |
5 |
|
Drancourt M Antim Agents Chemoter 1993 |
3 |
1 |
Table 2 Summary of different studies showing poor results in the use of suppressive antibiotic treatment alone
A patient selected for antibiotic suppressive treatment must meet following criteria (Table 3):
|
Indication for Antibiotic Suppressive therapy |
|
§ General contraindications to surgery |
|
§ Low virulence of causative microrganism |
|
§ Susceptibility of the germ to oral anticiotics |
|
§ The antibiotic must be tolerated without problems and general toxicity |
|
§ The prosthesis must be stable |
Table 3 Criteria used to decide to suppressive antibiotic treatment alone
Prolonged antibiotic suppression therapy should be employed according the culture results, obtained by intra-articular fluid aspiration of sinus tract sample. In this contest the infection disease Consultant can make essential contribution to the development and supervision of safe, effective, and cost efficient treatment plans. The choice of a specific antibiotic is dictated by antimicrobial activity, pharmacokinetics, tissue penetration and as we have already said potential toxicities of the antimicrobials under consideration. In case of Staphylococcus infection, the use of more than one antibiotic, such as Rifampicin and Fluorchinolones has been shown to potentially improve treatment outcomes and should take into consideration.34
Surgical debridement
When infection is diagnosed within 4 weeks of its appearance, prosthesis is stable and the patient's general condition is good, articular debridement may represent a reasonable treatment option.36,37 The clinical conditions in which surgical debridement is indicated are early post-operative infection and hematogenous acute infection (Table 4). In both cases the onset is acute, with important local inflammation and often systemic toxicity. The long term results of debridement surgery are difficult to assess, as the literature data differ in relation to the type of germ involved, the duration and mode of administration of antibiotics and the evaluation criteria. The positive results after debridement vary greatly.36–39 The major series, however, show that the success rate is around 30% of cases6,18,23,30(Table 5).
|
Indications for surgical debridement |
|
§ Acute hematogenous infection or early post-operative infection (within 4/6 weeks). |
|
§ Absence of draining skin sinus. |
|
§ Stable prosthetic components. |
|
§ Staphylococcus Epidermis or Streptococcus. § Staphylococcus Aureus (if surgery timing within 2 weeks). |
Table 4 Indications for surgical debridement
|
Author |
N° of cases |
Success cases (rate) |
FU (months) |
|
Woods GW Clin Orthop 1983 |
27 |
3(12%) |
n.r. |
|
Freeman MA JBJS Br 1985 |
6 |
5(83%) |
12-40 |
|
Johnson DP JBJS 1986 |
27 |
2(7%) |
33.6 |
|
Grogan TJ* JBJS 1986 |
4 |
3(75%) |
n.r. |
|
Flood JN* Arthroscopy 1988 |
2 |
2(100%) |
30 |
|
Borden LS J Arthroplasty 1990 |
11 |
5(45%) |
51 |
|
Teeny SM J Arthroplasty 1990 |
21 |
6(29%) |
42.5 |
|
Wilson MG JBJS 1990 |
31 |
17(55%) |
34 |
|
Schoifet SD JBJS 1990 |
31 |
7 (23%) |
105.6 |
|
Bengston S Acta Orthop Scand 1991 |
154 |
37(24%) |
72 |
|
Hartman MB Clin Orthop 1991 |
33 |
20(61%) |
54 |
|
Burger RR Clin Orthop 1991 |
39 |
7(18%) |
49.2 |
|
Kramhoft M J Arthroplasty 1994 |
27 |
6(22%) |
n.r.
|
|
Wasielewsky RC J Arthroplasty1996 |
9 |
6(67%) |
57 |
|
Wasielewsky RC* J Arthroplasty 1996 |
1 |
1(100%) |
57 |
|
Mont MA J Arthroplasty1997 |
24 |
20(83%) |
48 |
|
Segawa H JBJS 1999 |
10 prec 11 tard |
5(50%) 1(1%) |
48 |
|
Waldman BJ* J Arthroplasty 2000 |
16 |
6(38%) |
64 |
|
Deirmengian C J Arthroplasty 2003 |
35 |
11(35%) |
24-120 |
|
Ilahi OA* Arthroscopy 2005 |
5 |
5(100%) |
41 |
Table 5 Surgical debridement
Literature review (*, patients with arthroscopic debridement; nr, not reported)
Some factors, however, have to be discussed. The early intervention for many authors is the most important factor for success. In a 1990 study Teeny et al.37 have observed that after 2 weeks of symptom onset the chances of success have drastically reduced. The importance of the concerning germ was demonstrated by Schoifet and Morrey in 1990, who reported the results of 31 cases of debridement in the presence of acute infection treated at the Mayo Clinic in Rochester.36 The healing of the infection was obtained only in 7 cases with a mean follow-up of 8.8 years. All patients in whom the infection was supported by Gram negative had a recurrence of the infection. The S.Aureus was involved in 29% of cases, while he had infected 58% of failures. More recently Deirmengian et al.4 reported 12 cases of failure out of 13 patients who had infection with S.Aureus, while healing was 56% of cases when the causative agent was the S.Epidermidis or Streptococcus.
Debridement can be performed arthroscopically or through open arthrotomy. With the arthroscopic treatment have been reported positive results in selected patients treated within a few days after onset of symptoms.40 Arthroscopically, however, complete excision of synovial inflammation and/or infected tissue can be difficult in the presence of abundant scar tissue.41 Finally, it is impossible, with this method, to proceed to the replacement of the insert of polyethylene, which is however strongly recommended in these cases.6,18,23 The use of continuous washing with post-operative antibiotic solutions is controversial,42,43 but cannot find many supporters at the time.6 The debridement is not possible without adequate antibiotic treatment assessed in relation to the sensitivity of the infecting germ. The choice of the most effective drugs and the treatment duration is of relevance of infectious disease Consultant.
Prosthetic replacement
Reimplantation of a new prosthesis is obviously the desired preferred solution by the patients and the surgeons because it offers the possibility to re-establish the function of the knee. Even though eradication of infection should be considered as major goal of the treatment. Selection of patient for reimplantation correlates with several conditions, some are connected with the host whereas others are related to the characteristic of the infection and in particular with the virulence of the microorganism. Regarding the host condition, a patient with a deficient immune system or carrier of factors that diminished his/her metabolic, and/or hematopoietic capabilities should be considered a poor candidate for reimplatation. A useful staging prosthetic joint infections system designed to facilitate a comparison of outcomes in specific host cohorts has been proposed by Cierny and DiPasquale.44 According to this staging system the health condition of the patient is classified using parameters found previously which adversely affect wound healing. Systemic factors are malnutrition, immune deficiencies, chronic hypoxia, malignancies, diabetes mellitus, and extremes of age, chronic tobacco abuse and major organ failure. Local factors include chronic lymphedema, venous stasis, major vessel disease, arteritis, extensive scarring, radiation fibrosis, retained foreign bodies (suture, buckshot).
Others Authors noted a higher degree of failure in patients with rheumatoid arthritis compared with those with osteoarthritis or in case of diabetes mellitus.45 The increased predilection for infection is probably correlated with a greater tendency toward delayed or failed wound healing and diminished host resistance.46 This might be related to decreased neutrophils function47 and nutritional compromise. Regarding the nutritional status an albumin level of <3.5 g/dL, a total lymphocyte count of <1500, or a serum transferrin level of <262 mg/dL have all been found to increase the risk of infection, length of hospital stay, and mortality in elderly patients.48,49
Another important factor that to take into account is grading of the local wound. An intact soft tissue envelope with normal perfusion is critical to eradicating the infection at the local site. An intact vasculature is necessary to deliver oxygen, immune cells, and mediators to the area of infection. If the local wound is damaged significantly, then undamaged fresh tissue can be used to fill deficits and reestablish a vital local status; local muscle rotational flaps could be helpful in some cases (Figure 3). If there is poor extremity perfusion, the real possibility of success are extremely reduced
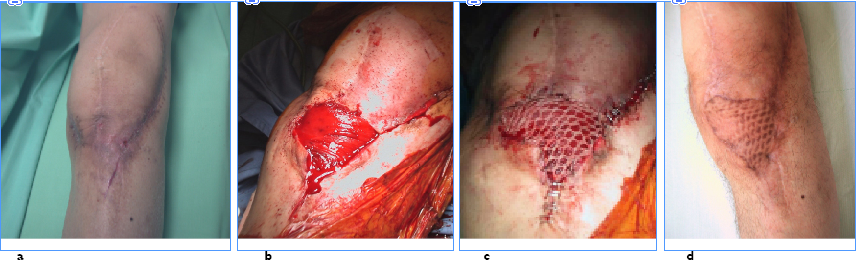
Figure 3 a) local wound aspect before antibiotic-loaded cement removal and reimplantation of a new prosthesis b) medial gastrocnemius flap coverage c) thiersch graft d) 1 year follow-up.
Prosthesis removal
The removal of prosthesis and surgical debridement is challenging and difficult. First at all is important to consider which surgical exposure to use before the operation. Isolated preexisting longitudinal approaches should be reused; in particular a well-healed medial parapatellar incision should always be used rather than making a new adjacent midline incision. In cases where more than one longitudinal incision are present is better to select the more lateral one (Figure 4), as blood supply entering from the medial side is significantly more than that entering from the lateral side. Revision incision should be long enough to provide wide exposure of distal femur and proximal tibia, and to allow extensive procedures if necessary. Intra-articular access could be in fact limited by a tight or compromised extensor mechanism. In these cases a lateral release or the snip of quadriceps tendon as described by Insall50 may be necessary to facilitate exposure. Patients with longstanding flexion contracture or extensile scars could require more aggressive exposure as tibial tubercle osteotomy.51 Once a sufficient exposure is obtained it is important to perform an extensive synoviectomy with debridement and removal of all prosthetic components and bone cement. The area of excision is sometimes widened slightly in areas where there are draining sinuses. The excision of all necrotic tissue is essential as well as complete excision of all pseudo membranes. As for aseptic revision, modular polyethylene is removed first in order to gain more space and reduce tension in the joint area. Even if could be difficult to remove a well fixed tibial component with the femoral component in place, attention should be paid to femur too. Thin osteotoms or small oscillating saw are ideal to get loose the femoral component before to use extraction tools or impactors. In case of extension stems, more elaborate procedures are needed especially in case of porous coated or cemented components, up to femoral transection (Figure 5). Osteotomes or saws are used to loosen the cement under a metal-backed tibial component. The removal is then completed by extractors or using the technique of stacked osteotoms.52 Reinsertion of a new prosthesis can then be performed as a direct exchange, the so called one stage reimplantation, or after a variable time delay, the two stage procedure.
One-stage prosthetic revision
One-stage revision of a total knee arthroplasty consists on the removal of the implants and the positioning of a new prosthesis during the same surgical session. This technique has been widely applied from Endo-klinik school of Hamburg since the seventies, who describe in detail the timing for the hip prosthesis replacement.53 The fundamental steps of this replacement are represented by: pre-operative needle-aspirate to identify germ and choose antibiotics with an experienced microbiologist advice; careful surgical removal of scar tissue, fistulas, necrotic tissue and all the cement’s fragments; finally cementation of the prosthesis with antibiotic-loaded bone cement chosen according to the antibiogram. In a recent literature review by Silva et al.54 were assessed 8 series with a total of 37 cases: the healing of infection was obtained in 34 of these patients (89.2%) (Table 6). Freeman has shown the largest series with the longest follow-up in 2 subsequent publications in 1985 and 1992:55,56 18 patients have been reported 2 failures related to a case of re-infection with the same germ and a case of new infection, both in patients suffering from rheumatoid arthritis. All cases of infection described by Freeman were caused by gram positive, were not present signs of systemic toxicity and were used cement added with gentamicin and the post-operative antibiotic treatment was prolonged for 3 months. Similar results were reported more recently by Buechel57 in 22 revisions were developed only two infections in one case by the same germs while in the other by different germs. Factors associated with the success of one-stage revision surgery seem to be (Table 7): gram-positive infection, absence of fistulas, use of the antibiotic-loaded bone cement, post-operative antibiotic therapy of at least 12 weeks.
|
Author |
N° of cases |
Success cases (rate) |
FU (months) |
|
Freeman MA JBJS Br 1985 |
8 |
8 |
12-40 |
|
Johnson DP JBJS 1986 |
2 |
0 |
33.6 |
|
Borden LS J Arthroplasty 1987 |
3 |
3 |
51 |
|
Teeny SM J Arthroplasty 1990 |
1 |
1 |
42.5 |
|
Goksan SB JBJS 1992 |
18 |
16 |
60 |
|
Hanssen AD Clin Orthop 1995 |
2 |
0 |
? |
|
Selmon GP J Arthroplasty 1998 |
1 |
1 |
? |
|
Buechel FF J Arthroplasty2004 |
22 |
20 |
122.4 |
Table 6 One-stage prosthetic revision
Literature review
|
Indications for one-stage prosthetic revision
|
|
§ GRAM + substained infections |
|
§ Absence of sinus tracks |
|
|
|
§ Use of antibiotic-loaded bone cement according to the antibiogram |
|
§ A course of parenteral antibiotics administered post-operatively for 12 weeks |
Table 7 Indications for one-stage prosthetic revision
Two-stage prosthetic revision
Two-stage revision arthroplasty is considered to be the most successful method of treating chronic infected total knee arthroplasties58–60 with reported success rates ranged from 37.1% to 100%.60,61–65 Hovelius e Josefsson in 1979 reported the first successful case of two-stage treatment of infected knee using a chain with gentamycin-loaded cement balls inserted into the cavity left by removal of the prosthesis.66 However John Insall has been accredited to be the one who standardized the technique61 which consists on: a) removal of the prosthesis and all cement from tibia and femur; b) debridement of soft tissues and bone; c) 6 weeks of parenteral antibiotic therapy maintaining a minimum serum bactericidal titer of 1:8; implantation of new total knee prosthesis. This three phase protocol has been shown to be an effective method of treatment for infected knee arthroplasty with reasonable expectation of a successful result on condition that all the phases are rigorously followed. The polyethylene tibial insert is removed along with tibial and femoral component, following the steps described before. The wound is thoroughly irrigated with abundant saline solution, and a spacer is formed with antibiotic- impregnated methyl methacrylate.
The treatment goals with the use of Antibiotic Impregnated Cement Spacer (AICS) is two-fold: one mechanical and the other biological. The AICS is useful to stabilize or tension the joint space. Potentially facilitate or make the reimplantation second stage easer. The second biological advantage of AICS is based on the possibility to deliver locally a high dose of antibiotic into the joint between the first and second procedure. The use of these spacers blocks was firstly reported in 1988 by Cohen et al.58 who treated three cases with this technique. The infection was controlled in all patients and the Authors were able to succefully implant a well functioning prosthesis. The AICS for total knee surgery are either static (spacer block) or mobile (Figure 6).
The last one may be commercially preformed with a fixed antibiotic dose or67 or prepared in the operating room through the use of special molds selecting the antibiotic of choice.68 According the use of antibiotic to mix into the cement there are large volume of documentation available in literature starting from the work of Buchholz69 and Cerretani70 particularly studied the association of a mixture of vancomycin (2g) and meropemen (2g) handled together with 40g of plain cement, as a result of increased presence of gentamycin resistant bacteria. From a general point of view the antibiotic should be: a) thermostable, as the polymerization of cement is an exothermic reaction that generates a substantial amount of heat; b) water-soluble, to permit diffusion into surrounding tissues while allowing a gradual release over time for a sustained bactericidal effect c) should have non systemic toxic effects d) should be available in powder.5 According to this last argument recently it has been reported by Seldes et al a paper where it was documented the possibility to use liquid gentamycin with the advantage of reduced cost.71 The spacer blocks are hand-made in the operating room and are sized to fit the defect created by removal of the infected prosthesis. The choice of antibiotics to add to the cement is based on recommendations of the infectious disease Consultant. Ideally part of the block should be fashioned as a peg in order to anchor it to the tibia and prevent its migration; moreover it is important to mould the spacer in anterior part of the femur so that avoid scarring between femur and extensor mechanism. With spacer block the knee is held in brace or cast and the weight bearing should no allowed because the risk of bone erosion and dislocation. Despite the assumption that spacer block could facilitate the second stage, revision procedure are still difficult and scarring tissue around the knee is nevertheless substantial. As the majority of the morbidity of this technique is related to the scarring and lack of motion between stages, new spacer have been developed in order to allow not only a distraction of the joint but also motion and weight bearing.
Attempts to overcome these problems led to the development of articulating spacer blocks such as the PROSTALAC system, which was first reported in middle nineties.72,73 The PROSTALAC spacer is an articulating prosthesis composed primarily of Palacos cement loaded, by the surgeon, with a combination of tobramycin and vancomycin per package. The femoral and tibial components are molded intraoperative into various sizes and thicknesses. The implant has a small metal-on-polyethylene articular surface. In 1995 Hofmann et al described a technique using the original knee component, after sterilization of the femur one, with a new tibial insert and patella. These were cemented in place with highly cured Palacos cement containing 3.6 g of tobramycin and 2 g of vancomycin per 40 g package cement. The articulating surface was polyethylene-on-metal, an optimal configuration for knee motion. More recently a pre-formed articulating spacer made of gentamicin-impregnated acrylic cement has been reported.74
Based on a review of the literature, the addition of a small amount of metal and plastic to antibiotic bone cement have not seem to have an adverse effect on infection cure rate and has the advantage of improved function between stages.68,73,75,77 Despite its widespread acceptance, two-stage revision has several controversial aspects, including the timing of the procedure with correct evaluation for the reimplantation time and the use of antibiotic-loaded cement at the second stage (Table 8). Most protocols for delayed reimplantation include several weeks of intravenous antibiotic therapy. There has been some debate over the optimal duration for the period of IV antibiotic administration. The antibiotic chosen should be the most effective against the isolated organism and the least toxic for the host. It is important that close monitoring be performed to maintain serum levels in therapeutic range.60
|
§ Infection from high-virulence microorganism or polimicrobic infection |
|
§ Immunocompromised patients or patients with bad general conditions § Early revision (<6 weeks) § Prosthetic component cementation without antibiotic § Bad skin local condition |
Table 8 Negative prognostic factors during two-stage revision surgery
The majority of reports using 6 weeks have shown this to be effective. A controversial issue in field of two stage revision is related with correct choice to perform the second stage been sure that the infection is cured. Insall et al61 recommended that antibiotic therapy be stopped and then aspiration be performed before revision artrhroplasty. Others54 advocate cultures of the knee aspirate 4 weeks after stopping the antibiotics and before second stage re-implantation. We believe that this is unnecessary as a routine and should be restricted to those patients with a suspicion of persistent infection and in the presence of elevated inflammatory markers. The monitoring of serological test (CRP, ESR and fibrinogen) could be useful to detect that the knee is free of infection and its possible to perform a final procedure. In our experience the combination of at least two of three tests with values higher than the cut-offs is reliable for predicting the infection.78 Scintigraphy, needle-aspirate cell count and culture can integrate the pre-operative evaluation in Doubtful cases. Once infection control is established the second stage re-implantation is performed and multiple tissue samples should be taken at the second stage procedure. Even if the use of uncemented prosthesis at the second stage has been questioned at the light of recent promising result,79 the use of antibiotic-loaded cement at revision has been shown to reduce the rates of re-infection (Table 9). Hanssen and Rand80 had a success rate of 82% without antibiotic cement at the second stage procedure, compared with 90% when an antibiotic was used. Nowadays most surgeons prefer to use cement at the metaphyseal area with cementless stems.
|
Author |
N° of cases |
Success cases (rate) |
FU (months) |
|
Insall JN JBJS 1983 |
*11 |
8(72,7%) |
36 |
|
Borden LS J Arthroplasty 1987 |
*11 |
10( 90,9) |
51 |
|
Teeny SM J Arthroplasty 1990 |
*10 |
10(100%) |
42.5 |
|
Windsor RE JBJS 1990* |
*38 |
34(89,4%) |
4 8 |
|
Wilson MG JBJS 1990 |
*20 |
16(80%) |
34 |
|
Kramhoft M J Arthroplasty 1994 |
*15 |
11(73,3) |
n.r. |
|
Wasielewsky RC J Arthroplasty 1996 |
*6 prec *44 tard |
6(100%) 40(90,9%) |
57 |
|
Mc Pherson Clin Orthop 1997* |
* 21 |
20(95,2%) |
|
|
Hirakawa K J Arthroplasty 1998 |
55 |
41(74,5%) |
61 |
|
Segawa H JBJS 1999 |
29 |
24(83%) |
48 |
|
Fehring TK Clin Orthop 2000 |
* 25 ** 15 |
22(88%) 15(100%) |
36 27 |
|
Emerson RH Clin Orthop 2002 |
* 26 ** 22 |
18 (69.3%) 20 (90.9%) |
90 45 |
|
Meek RMD JBJS 2003 |
**47 |
45(96%) |
47 |
|
Hallem AA Clin Orthop 2004 |
96 |
87 (91%) |
90
|
|
Durbhakula J Arthrop 2004** |
**24 |
22(92%) |
33 |
|
Hoad-Reddick DA JBJS (Br) |
**38 |
34(89%) |
56 |
|
Hofmann A Clin Orthop 2005 |
**50 |
46(92%) |
73 |
Table 9 Two-stage septic revision surgery
Literature review (*, spacer block; **, articulating spacer).
Arthrodesis
Arthrodesis is a salvage procedure for an infection of total knee arthroplasty, that aims to achieve stable and painless joint despite a complete loss of motion.6,81,82 In case of knee arthroplasty septic failure the indications for arthrodesis can be summarized as: highly resistant infection to antibiotic therapy, extensor deficits, poor skin coverage that can not be adequately reconstructed, younger age, and immunodeficiency.6,11,17,60,83 Relative contraindications to arthrodesis are ipsilateral hip or ankle pathologies, extensive bone loss, and contralateral amputation.6,11,60 Different surgery procedures have been proposed to obtain knee arthrodesis, but at this time external fixation81,84,87(Table 10) and the intramedullary synthesis6,85,86 (Table 11) are the most frequently used techniques.
|
Author |
N° of cases |
Fusion rate |
|
KnutsonK JBJS 1984 |
71 |
33(46%) |
|
Green SA Orthop1985 |
7 |
3(43%) |
|
Hagemann JBJS 1986 |
14 |
9(64%) |
|
Rand J JBJS 1987 |
28 |
20(71%) |
|
Hak DJ 1Clin Orthop1995 |
22 |
12(55%) |
Table 10 Femoral-tibial arthrodesis by external fixator
Literature review
|
Author |
N° of cases |
Fusion rate |
|
KnutsonK Clin Othop 1984 |
10 |
9(90%) |
|
Harris C Clin Orthop 1985 |
8 |
7(88%) |
|
EllingsenDE JBJS 1986 |
18 |
16(89%) |
|
Puranen J JBJS 1990 |
15 |
12(80%) |
|
DonleyBG JBJS 1991 |
9 |
8(89%) |
|
FerroneJD Comp. Orthop 1996 |
8 |
8(100%) |
|
Waldman BJ Clin Orthop1999 |
21 |
20(95%) |
|
Incavo SJ J Arthrop 2000 |
17 |
17(100%) |
Table 11 Femoral-tibial arthrodesis by intramedullary nail
Literature review
The problems associated to this type of arthrodesis, regardless of the surgical choice, are related to the entity of bone loss, bone shortening, alteration of deambulation and finally the possible persistence of knee infection.6,85,86 The percentage of success with external fixation varies widely according to the characteristics of the device used (Figure 7). In the early 80's, the success rate observed with monoplanar external fixator was poor with union in only 1/3 of cases.85,86,88 Enhancement of fixator stability increased the union rate reaching values of 68% with biplanar systems.86 On the contrary Hak et al. showed only a small difference in union percentage by changing from monoaxial to biplanar fixator.
These data suggest that the bone union depends both on the fixator stability and by other factors, such as the type of prosthesis previously used which may influence the extent of bone loss and the bone quality, and consequently the bone contact and the possibility of compression. The best results have been observed after failure of resurfacing prosthesis compared with hinge implants, especially with intramedullary stems87 because the bone contact and compression of the articular heads are limited in the presence of cortical or sclerotic bone.
The arthrodesis by intramedullary nail (Figure 8) has gained increasing favour, due to rates of fusion greater than 80% of cases,86,89,90 despite the literature is often based on heterogeneous series of cases secondary to septic or aseptic loosening and bone resection for malignancy.6,91,92
Waldman et al.41 showed the results of a multicenter study on 21 patients treated by modular titanium nail for septic knee arthroplasty loosening followed for 2,4 years (2 to 7.5 years): a solid union was obtained in 20 of 21 patients about 6,3 months after surgery. The authors underline the advantages of this method in contrast to the traditional technique that included the introduction of a long nail from the trochanteric region. The modularity and the morphology of the nails allow it to adapt to different dimensions of the femoral and tibial canal and to facilitate the alignment of the limb. In the presence of bone loss is possible the use of Titanium spacers or bone grafts. Although no patients in this series developed a new infection, the possibility that this event will occur is high, due to the presence of nail. For this reason a two stages protocol is recommended: the initial removal of the prosthesis is followed by nail arthrodesis.82–90
Resection arthroplasty
The implant removal without the intention to further positioning of a new prosthesis was restricted to situations of serious infections in debilitated patients. Although the procedure was considered effective for infection healing, the consequent knee pain and instability, and the difficulty in walking makes it difficult to use. Now-days it should be considered only as exceptional treatment.
Amputation
Above knee amputation or rarely hip disarticulation is a salvage procedure for the failed infected total knee arthroplasty. The indication for amputation is life threatening sepsis particularly if associate with massive bone loss. Amputation with prosthetic fitting will allow the most rapid rehabilitation and avoid the morbidity of additional surgical procedure. However it should take into account that many of elderly patients may became limited ambulators or no ambulatory for psychological or objective difficulty.

©2018 Amendola, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.