MOJ
eISSN: 2374-6939


Mini Review Volume 9 Issue 2
1Assistant Professor, Department of Rheumatology, Saint-Joseph University, Lebanon
2Consultant Rheumatologist, Hotel-Dieu de France Hospital, Lebanon
Correspondence: Nelly Raymond Ziade, Department of Rheumatology, Assistant Professor, Saint-Joseph University, 6th floor, Tour des Consultations Externes, Hotel-Dieu de France hospital, Alfred Naccache blvd, Achrafieh, Beirut, Lebanon, Po Box 166830, Tel 9611615304
Received: July 19, 2017 | Published: September 9, 2017
Citation: Ziade NR (2017) Registries in Spondyloarthritis: What have we Learned? MOJ Orthop Rheumatol 9(2): 00347. DOI: 10.15406/mojor.2017.09.00347
Registries are becoming increasingly useful in understanding disease outcomes at the population level and on the long-term, especially for chronic rheumatic diseases such as spondyloarthritis. This article introduces the recommended methodology for an optimal registry, discusses the values of registries in rheumatic diseases and reviews the main spondyloarthritis registries worldwide.
Keywords: Spondyloarthritis, Registry, Cohort, Epidemiology
A registry is defined as “an organized system that uses observational study methods to collect uniform data to evaluate specified outcomes for a population defined by a particular disease, condition, or exposure, and that serves predetermined scientific, clinical, or policy purpose(s)”.1
A registry is a prospective, systematic protocol-driven approach to data acquisition. The main advantage is that it can capture large numbers of patients across large geographic zones and assess several outcomes for a prescribed cohort of patients. It can serve as a valuable resource for patient advocacy, patient education and support, incidence and prevalence, broad demographic profiles and establishment of classification criteria.
An optimal registry is expected to2
The methodology for establishing a registry comprises several steps2 Before date collection ensues, a group of individuals (organizing committee) must come together to clearly establish the underlying purpose of the registry to be established, which investigators will constitute the leadership and oversight of the registry, who will be responsible for obtaining funding, and how the data will be collected. This committee will also decide who will participate in the data collection and monitoring and which criteria will be used for patient recruitment and data entry, as well as inclusion/exclusion criteria, and mode of data collection. Power issues relative to the endpoints being examined should be defined upfront and an appropriate sample size established. The endpoints and data points should also be realistic, collectable within the study budget and feasible in the setting of where the data will be extracted (clinics, insurance records, etc.). Issues surrounding retention in and adherence to the registry should be addressed, and strategies to deal with the missing data planned upfront.
Registries can be classified in two types2
The longitudinal registry may include a fixed cohort of all disease cases (as the SPARTAN7), a fixed cohort of early disease cases (inception cohort, as the DESIR,8 GESPIC,9 SPACE10 or ASAS11) or may be dynamic with continuous inclusion (as the CORRONA12).
In some cases, historical cohorts are used, such as the OASIS cohort13 used for baseline data from the pre-biologic era. Some registries are combined to form one bigger registry (as the North American registries PSOAS,14 PULSAR15 and SPARCC.16 And finally, some registries are more oriented towards cost-effectiveness evaluations at the academic (SpA-Net17) or national governmental level (Korean registry18).
Since the biopharmaceutical revolution witnessed in the treatment of the inflammatory rheumatic diseases during the last two decades, the need to develop registers at national and international levels has become obvious. In particular, biologics registers have provided robust and reliable evidence for the effectiveness and safety of these therapies in real life setting, based on greater number of patients followed for longer periods of time than clinical trials.19 The inflammatory rheumatic diseases’ natural history distinguishes them from other diseases such as cancer, and their chronic nature and necessity for long term therapy has mandated the establishment of registries to monitor the long-term efficacy and safety in a real-life setting and provide data that cannot be obtained through clinical trials. In this aspect, data obtained from unselected patients provides good external validity and generalizability.20-27
For example, biologics registries have provided invaluable data about treatment switching and long-term drug retention rates, which reflects efficacy, safety and adherence to the drug.28-30 They helped elucidate the very debated association between biologics and malignancies and provided solid reassurance about this major safety issue.31-32
Registers allowed the study of comorbidities in real life, whereas in clinical trials patients with major comorbidities are excluded; they elucidated the notion of multimorbidity patterns.33
They shed the light on country specificities such as the rate of biologics use, the level of education and the presence of disabilities. And finally, biologics registers reflected somehow disease registers, such as in the UK, where any rheumatologist prescribing biologics for rheumatoid arthritis has the obligation to register their patients with the British Registry BSRBR.21
However, registers still face many challenges such as long-term sustainability due to their significant cost, multiple biases -mainly confounding factors- due to the lack of randomization and international comparability due to different methodologies and the use of different variables.19,34
The major SpA based registries are listed below (by alphabetical order), with a description of the methodology and the population included and a brief report of findings. Most of the data that we have today come from Europe and the Americas. Figure 1 presents the major SpA registries by geographic distribution, dates and number of patients.
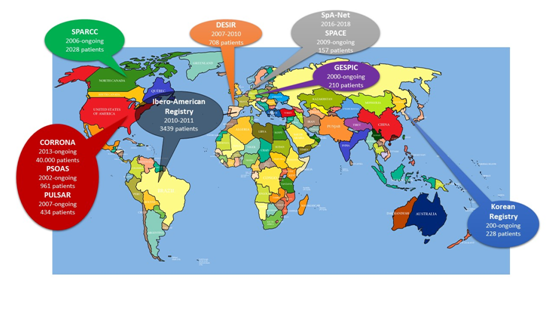
Figure 1 Spondyloarthritis disease-based registries across the world: geographical distribution and basic information.
The dates below each registry are the start and end dates of the registry, respectively. The number of patients included is mentioned for each registry.
ASAS (Assessment of Spondyloarthritis International Society) cohort11
The ASAS cohort was compiled for the validation of the new classification criteria for AxSpA. Patients with chronic back pain of at least 3 months with onset less than 45 years and with a suspicion of SpA but without a definite diagnosis were included and assessed according to a fixed protocol by rheumatologists who are experts in the field of SpA. Assessments included past or current SpA features, C-reactive protein, and HLA-B27 typing. Plain radiographs of the pelvis were taken in all patients. The local rheumatologist and radiologist assessed sacroiliitis on standard radiograph and the presence or absence of typical signs of active inflammation on pelvic MRI. It resulted in the development of ASAS classification criteria for axial spondyloarthritis.
CORRONA (Consortium of Rheumatology Researchers of North America (Johns Hopkins))12
CORRONA is a prospective, multicenter, observational registry for patients with spondyloarthritis and psoriatic arthritis. It has longitudinal follow-up information via data collected from both patients and their treating rheumatologists every 6 months. Patients are enrolled during regularly-scheduled office visits. Selected rheumatologists are invited to participate as investigators in the Registry. Physicians are selected carefully in an effort to ensure enrollment of subjects that represent a reasonable representation of a cross-section of the population throughout the country with rheumatic diseases. The inclusion criteria are male and female patients with all forms of SpA, including those who meet the 1984 Modified New York Criteria for Ankylosing Spondylitis, the 2009 ASAS Criteria for Axial Spondyloarthritis, and the 2010 ASAS Criteria for Peripheral Arthritis or the CASPAR for psoriatic arthritis. Patients with other forms of overlapping non-autoimmune arthritis, such as osteoarthritis or gout, are allowed, but will have these forms of arthritis clearly distinguished from their SpA manifestations. The primary goal of the registry is to systematically collect and analyze longitudinal outcomes associated with spondyloarthritis. This includes assessing baseline characteristics, and trends in patient characteristics and treatment patterns. CORRONA evaluates prevalence rates of comorbidities and the burden of disease in the population. The registry is dynamic and creates a national cohort of Spondyloarthritis patients (40.000) in real world setting cared for by rheumatologists to better understand the epidemiology and natural history of the disease, comorbidities, current treatment practices, response to therapy and outcomes related to medication therapy. The large dataset provides information about safety, effectiveness, pharmacovigilance and provides insights to treatment decisions.
DESIR (Devenir dEs SpondyloarthropathIes Récentes)8
DESIR is a national, French, multicenter, longitudinal, prospective follow-up of patients aged 18-50 years. It includes 25 regional centers in France presenting with early inflammatory back pain (IBP). It is established by the French Society of Rheumatology in order to set up a database to facilitate several investigations on diagnosis, prognosis, epidemiology, pathogenesis, and medico-economics in the field of early IBP and SpA. It includes IBP of more than 3 months and less than 3 years, and are followed every 6 months during the first 2 years then every year during at least 5 years. Data collected include demographics, disease activity, severity, comorbidities, socio-economics, treatments, radiological and MRI evaluation of the spine and the pelvis according to the local investigators. The recruitment period of the 708 patients in the 25 centers was 26 months (from December 2007 to April 2010). It allowed the evaluation of the long-term evolution of IBP, the role of prognostic factors such as smoking… and correlations between clinical and radiological features.
GESPIC: German Spondyloarthritis Inception Cohort9
GESPIC is an ongoing, prospective, longitudinal study. It examines clinical, functional, and structural outcome of SpA of short duration. It was set up in 2000 as part of the German Competence Network Rheumatology program. It involves four university hospitals, five community hospitals, and four private practices. The main inclusion criteria are definite clinical diagnosis of axial SpA according to the treating rheumatologist. Patients with axial SpA were further classified by the local rheumatologist based on the radiographic findings as having either AS or non-radiographic axial SpA (modified New York criteria): 210 patients (95 NRX + 115 AS). The duration of symptoms was restricted to or less than 10 years at the time of inclusion. It allowed the study of clinical (male gender) and biological (CRP) factors on radiographic progression and further characterization of the non-radiographic form of SpA.
The registry is cross-sectional and includes data from: RESPONDIA (The Ibero-American Registry of Spondyloarthritis): 100 university centers from 10 countries and REGISPONSER (Spanish Society of Rheumatology (SER)). It involves 3439 patients. The objective is to collect demographic data, clinical data, and quality of life. The main addition is the finding of very large clinical differences, perhaps reflecting differing referral patterns or clinical foci of the contributing sites.
OASIS (Outcome Assessments in Ankylosing Spondylitis International Study)13
OASIS is an international, longitudinal, observational historical cohort on outcomes in AS. It comprised 217 consecutive Dutch, French, and Belgian patients with AS established in 1996 and followed into the next decade. All patients were TNF-Naïve, most having been treated primarily with NSAIDs alone and were felt to represent the spectrum of AS seen in practice at the time. The OASIS cohort was used as the comparison cohort for assessing the radiographic progression in most of the anti-TNF trials.
PSOAS (Prospective Study of Outcomes in Ankylosing Spondylitis)14
PSOAS is a NIH funded study based at four academic medical centers in the United States and followed patients longitudinally since 2002. As of January 2013, 961 patients had been enrolled of whom over 600 are still being longitudinally followed, with extensive standardized clinical, psychological, and sociodemographic instruments applied at each study visit, and genetic, serologic, and radiographic data collected and analyzed.
PULSAR (Program to Understand the Longterm Outcomes of Spondyloarthritis)15
PULSAR was initiated in 2007 to better characterize the clinical and pathophysiologic aspects of SpA in U.S. veterans. 434 patients have been enrolled (mean age 59.1 years, 93% men, 72% white, 31% HLA-B27 positive, 23% AS, 35% PsA, 6% with uveitis, 3% IBD-associated SpA, 2% undifferentiated spondyloarthritis, and 42% on anti-TNF agents).
SPACE (Spondyloarthritis Caught Early cohort)10
SPACE is an observational inception cohort including patients with chronic back pain, since January 2009. Inclusion criteria are patients with chronic (almost daily) back pain for 3 months - 2 years, at the outpatient clinic in the Leiden University Medical Centre. Patients undergo MRI and radiograph of the sacroiliac joints at baseline. Patients were diagnosed as having axSpA if they had IBP (by ASAS expert criteria and at least three SpA features) or if patients had IBP with one or two SpA features and were HLA-B27 positive. Patients with no other SpA features in addition to IBP could only be diagnosed as having axSpA if both HLA-B27 and active sacroiliitis (MRI-SIJ) were present. Many clinical and radiographic outcomes are derived.
SpA‐Net (Spondyloarthritis register in the Netherlands)17
The methods use a web-based patient medical file of unselected SpA patients and simultaneous quality management system. The main objectives are to study patient outcome and pharmacovigilance and personalised medicine with cost-effectiveness background. The study period is 2016 – 2018.
SPARCC (Spondyloarthritis Research Consortium of Canada)16
SPARCC is launched by a transdiscliplinary research network of investigators interested in spondyloarthritis. The aim was to address the genetic basis of susceptibility of the disease and develop and validate clinical and imaging outcomes to assess disease activity and structural damage over time, the response to therapy, and the clinical burden of illness in terms of quality of life and disability. It studied 2028 patients and generated several indices (SPARCC enthesitis index, SPARCC imaging scoring system…)
SPARTAN (Spondyloarthritis Research and Treatment Network)7
SPARTAN is a registry under the umbrella of scientific societies in North America. It combines data from PSOAS, PULSAR and SPARCC.
The Observation Study of Korean Spondyloarthropathy Registry cohort18
It is an ongoing, longitudinal, observation study. It studies clinical, functional, and structural outcome of spondyloarthritis in Korea and is supported by the Korean Ministry for Health, Welfare, and Family Affairs. It includes patients meeting the modified New York criteria for AS. Clinical data include age, sex, duration of disease, age at onset of AS symptoms, family history of AS, history of uveitis and iritis, peripheral arthritis, enthesitis, and HLA-B27 carrier status, as well as functional capacity (BASFI).
For better comparison, the registries characteristics are summarized in Table 1, with a focus on geographic areas, dates on study, number of patients, patients’ profiles, methodological features and key findings.
|
Registry |
Region |
Dates |
Number of Patients |
Patient Profile |
Methodology |
Endpoints |
Key finding |
|
ASAS Cohort |
Germany (Charité Campus |
2008 |
71 patients |
Patients with chronic back pain of at least 3 months with onset less than 45 years and with a suspicion of SpA but without a definite diagnosis |
Retrospective review of charts of difficult cases by 20 ASAS experts |
SpA features, C-reactive protein, HLA-B27 typing, Sacroiliitis on plain radiographs and MRI |
Validation of the new ASAS classification criteria for AxSpA |
|
CORRONA |
USA |
2013 - |
40.000 patients |
All forms of Spa, enrolled during regularly-scheduled office visits by carefully selected rheumatologists |
Prospective, multicenter, observational registry, with dynamic longitudinal follow-up information via data collected from both patients and their treating rheumatologists every 6 months. |
Baseline characteristics, trends in patient characteristics and treatment patterns, prevalence rates of comorbidities and burden of disease in the population |
Longitudinal outcomes in a real-world setting. |
|
DESIR |
France |
26 months (December 2007 to April 2010) |
708 patients |
Patients aged 18-50 years presenting with early inflammatory back pain, of more than 3 months and less than 3 years, and followed every 6 months during the first 2 years then every year during at least 5 years |
Prospective multicenter (25 centers), national, longitudinal |
Demographics, disease activity, severity, comorbidities, socio-economics, treatments, radiological and MRI evaluation of the spine and the pelvis. |
Diagnosis, prognosis, epidemiology, pathogenesis, and medico-economics in the field of early IBP and SpA. |
|
GESPIC |
Germany |
2000 - Ongoing |
210 patients (95 NRX + 115 AS) |
Definite clinical diagnosis of AxSpA according to the treating rheumatologist. |
Prospective, longitudinal (Four university hospitals, five community hospitals, and four private practices) |
Clinical, functional, and structural outcome of SpA of short duration. |
Study of clinical (male gender) and biological (CRP) factors on radiographic progression and further characterization of the non-radiographic form of SpA. |
|
Spain and South America |
2010-2011 |
3439 patients |
All Spa patients from tertiary healthcare centers (100 university centers from 10 countries in South America + Spain) |
Cross-sectional data from: RESPONDIA (The Ibero-American Registry of Spondyloarthritis) and REGISPONSER (Spanish Society of Rheumatology (SER)). |
Demographic data, clinical data, and quality of life |
Finding of very large clinical differences, reflecting differing referral patterns or clinical foci of the contributing sites |
|
|
OASIS |
Europe (Dutch, French and Belgian) |
1996 - 2006 |
217 patients |
Patients with AS, all TNF-Naïve, most having been treated primarily with NSAIDs alone |
Longitudinal, observational historical cohort |
Clinical, biologic and radiographic features |
Used as the comparison cohort for assessing the radiographic progression in most of the anti-TNF trials |
|
PSOAS* Prospective Study of Outcomes in Ankylosing Spondylitis14 |
USA - NIH funded study |
2002 -ongoing |
961 patients |
AS patients |
Longitudinal (four academic medical centers) |
Clinical, psychological, and sociodemographic instruments applied at each study visit, and genetic, serologic, and radiographic data |
Disease characteristics |
|
PULSAR* |
USA |
2007 |
434 USA SpA veterans |
SpA in U.S. veterans |
Observational, longitudinal |
Clinical manifestations and treatments |
Disease characteristics, extra-articular manifestations, treatment profile |
|
SPACE |
The Netherlands (Leiden University Medical Centre) |
January 2009 |
157 patients |
Patients with chronic (almost daily) back pain (CBP) for 3 months - 2 years, at the outpatient clinic |
Observational inception cohort including patients with chronic back pain |
Patients undergo MRI and radiograph of the sacroiliac joints at baseline |
AxSpA diagnosis in CBP. |
|
SpA‐Net: Spondyloarthritis register in the Netherlands17 |
The Netherlands |
2016-2018 |
Ongoing |
Unselected SpA patients |
Web-based patient medical file and simultaneous quality management system. |
Disease and treatment features, cost-effectiveness |
Patient outcome, pharmacovigilance and personalized medicine with cost-effectiveness background |
|
SPARCC* |
Canada |
2006-ongoing |
2028 patients |
All SpA patients |
Observational, longitudinal |
Genetic basis of susceptibility of the disease, clinical and imaging outcomes, disease activity, structural damage, response to therapy, clinical burden of illness |
Generated several indices (SPARCC enthesitis index, SPARCC imaging scoring system…) |
|
Observation Study of Korean Spondyloarthropathy Registry Cohort18 |
Korea |
2000-ongoing |
228 patients |
AS patients (modified New York criteria) |
Ongoing, longitudinal, observational |
Demographics, clinical, functional, HLA-B27, BASFI and structural outcomes |
Disease characteristics |
Table 1 Spondyloarthritis disease registries: summary of location, dates, methodologies and key findings.
*SPARTAN (Spondyloarthritis Research and Treatment Network) is a registry under the umbrella of scientific societies in North America. It combines data from PSOAS, PULSAR and SPARCC. AxSpA: Axial Spondyloarthritis.
Multiple types of registries exist worldwide. Their aims are different and they have helped the scientific committee to reach a wide range of objectives
Whatever the aim of registry is, the key to success is to establish clear goals upfront, to define the eligibility criteria for the included population, to carefully select the centers, to identify a minimum core set of variables to be collected to ensure data completeness and comparability across studies, and to train the rheumatologists for a standardized data collection. Sample size calculation is crucial to significant findings and patient implication and education may increase data completeness and quality. And finally, ethical and legal aspects of data handling and storage must be considered to ensure the security of patient’s identifiable information and compliance with local legislation in relation to data handling and storage.
None.
Authors declare there is no conflict of interest in publishing the article.

©2017 Ziade, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.