MOJ
eISSN: 2374-6939


Case Report Volume 6 Issue 5
Department of Orthopedic Surgery, Spine and Scoliosis Service, Hospital for Special Surgery, Weill Cornell Medical College, USA
Correspondence: Federico P Girardi, Department of Orthopedic Surgery, Spine and Scoliosis Service, Hospital for Special Surgery, 535 East 70th Street, New York, NY 10021, USA, Tel 212-606-1559, Fax 212-774-2870
Received: September 29, 2016 | Published: December 8, 2016
Citation: Salzmann SN, Lampe LP, Shue J, Moawad MA, Aichmair A, et al. (2016) Rapid Vertebral Osteolysis after Utilization of rhBMP-2. MOJ Orthop Rheumatol 6(5): 00238. DOI: 10.15406/mojor.2016.06.00238
Recent concern regarding the safety of recombinant human bone morphogenetic protein-2 (BMP-2) in spine surgery has increased awareness of a possible broad spectrum of adverse sequelae of this biologic. However, the impact of BMP-2 on postoperative osteolysis in the setting of lateral lumbar interbody fusion needs to be comprehensively evaluated. Three cases of significant osteolysis within the vertebral body following lumbar surgery supplemented by BMP-2 are presented. A detailed overview of one of the three cases is expanded on for the reader. Despite multiple prior reports, the incidence and spectrum of adverse clinical sequelae related to BMP-2 in the setting of spinal fusion surgery remains to be fully elucidated. Reporting of this delayed complication attributable to BMP-2 use will add to current reports on BMP-2 safety and complications. This case report will increase awareness of the possibility of post-operative osteolysis due to BMP-2 utilization.
Keywords: Bone morphogenetic protein-2, BMP-2, Osteolysis, Side effect, Complication, Lateral lumbar interbody fusion
rhBMP-2: Recombinant Human Bone Morphogenetic Protein-2, ICBG: Iliac Crest Bone Graft, LLIF: Lateral Lumbar Interbody Fusion, CT: Computed Tomography
Recently controversy has arisen regarding the safety of recombinant human bone morphogenetic protein-2 (BMP-2) in spine surgery. Multiple studies have reported use of BMP-2 to avoid autograft donor site morbidity.1,2 even in off-label use for spinal arthrodesis.3 The increasingly widespread use of this adjunct has been accompanied by several reports of adverse reactions to BMP-2, including neurological deficits.4-9 and the potential of BMP-2 to form morbid heterotopic bone.10,11 A series of 1,037 patients, undergoing posterolateral fusion utilizing BMP-2, quantified complications thought to be directly related to BMP-2 at 0.1-0.6%.7
Less invasive techniques such as lateral lumbar interbody fusion (LLIF) often are supplemented by biologic agents to facilitate intervertebral body fusion. Although BMP-2 use may be associated with high fusion rates, concomitant adverse reactions remain a source of concern, even with low doses.12
A recent systematic review reported that many industry trials may contain a bias favoring the safety and efficacy of BMP-2 while overestimating the morbidities of conventional iliac crest bone graft (ICBG).4 Since bone morphogenetic proteins function as growth factors, concerns for other adverse sequlae such as an increased cancer risk associated with BMP-2 use have been expressed.
Despite multiple prior reports, the incidence and spectrum of adverse clinical sequelae related to biologic agents in the setting of spinal fusion surgery remains to be fully elucidated. This case report raises awareness of post-operative osteolysis related to the utilization of BMP-2. This potential adverse effect of the adjunct in the setting of spine surgery should be carefully considered. Reporting this delayed complication attributable to BMP-2 use will add to reports on BMP-2 safety and complications.
All 3 cases are presented with available detailed computed tomography (CT). All patients underwent multilevel standalone LLIF procedures, without posterior instrumentation. Fusion cages were tempered with BMP-2 collagen sponges. The total administered doses of BMP-2 were 6 mg and 4 mg per level in a 2-level and 3-level surgery, respectively.
The main highlighted case reports on a 33-year old patient who underwent 2-level standalone LLIF (L3-L5), augmented with 6 mg BMP-2 per level. CT images at 4-months and 15-months postoperatively are shown and depict osteolytic bone destruction affecting the L5 vertebral endplate potentially related to the utilization of BMP-2 (Figure 1). Stability was not preserved and posterior instrumentation was required at the L4-L5 level 16 months after the initial surgery.
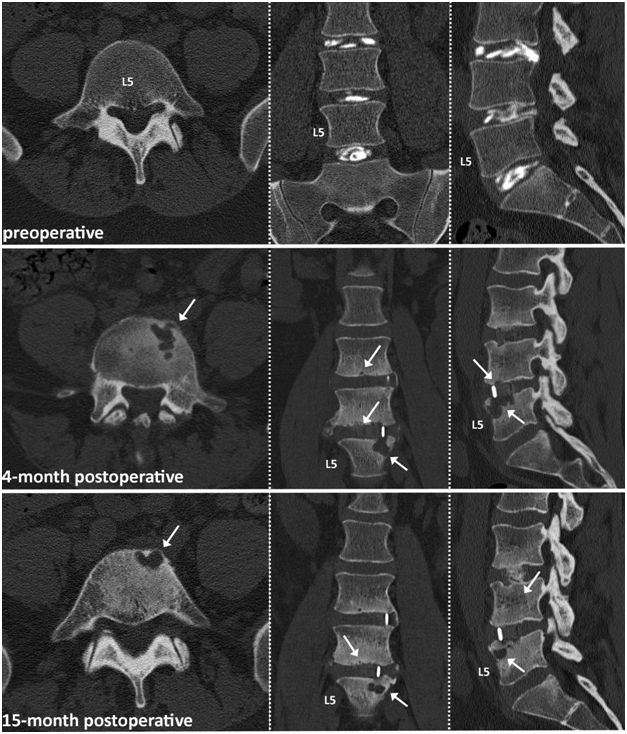
Figure 1 33-year old male patient with 2 level LLIF procedure at L4-5. White arrows indicating osteolytic bone destruction starting at the L5 vertebral endplate slightly improving after 15 months of watchful waiting.
At the time of the reporting, stability was preserved in the remaing two patients and they did not require further surgical treatment to address the osteolytic destructions (Figures 2&3). Total follow-up times for patients were 29, 22, and 6 months, for patient 1, 2, 3 respectively.
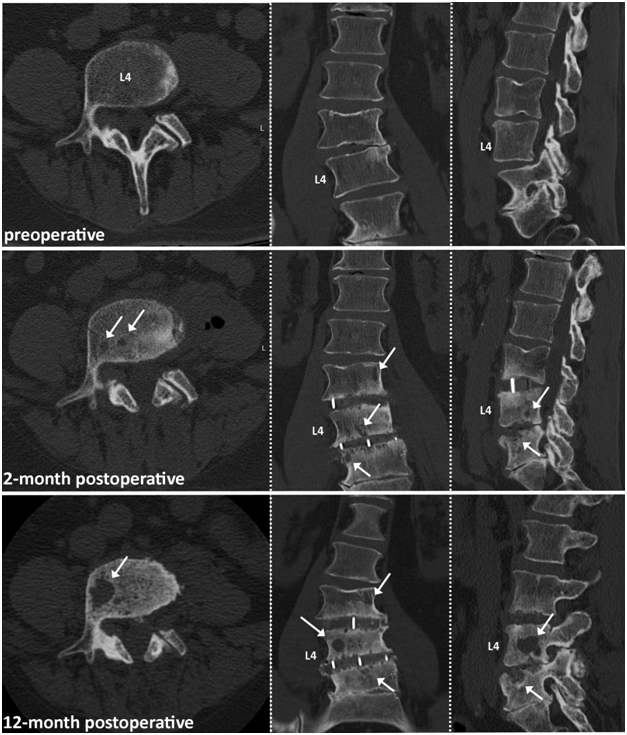
Figure 2 64-year old male patient with 2 level LLIF procedure. Note: Patient underwent posterior decompression 6 month prior to LLIF surgery. White arrows indicating starting osteolytic bone destruction at the L3-L4 and L4-L5 vertebra endplates 2 month postoperative. Osteolysis increased over the 12-month postoperative course, with one major big osteolytic cyst in the L4 vertebra.
Lumbar fusion is the most frequently performed surgery for the treatment of chronic back pain with lumbar disc disease.13 An important objective in this procedure is to obtain a solid arthrodesis using osteoinduction to promote spinal fusion.10,13 Besides ICBG (the current gold standard), other bone graft substitutes and enhancers like bone morphogenetic proteins are available.10
In 2002 the Food and Drug Administration (FDA) approved the use of rhBMP-2 with an absorbable collagen sponge carrier together with lordotic tapered cages in anterior lumbar interbody fusion procedures. Since receiving FDA approval, rhBMP-2 has been used extensively and for off-label indications.2
Utilization of rhBMP-2 is associated with high fusion rates without the need of harvesting autologous bone material from the iliac crest which is accompanied by lengthening of the surgical procedure and increased blood loss.1,10 Other complications include local pain, nerve or arterial injury, hematoma, infection and sacroiliac joint instability and are described to occur at an incidence of up to 25%. Furthermore the amount of bone harvested from the iliac crest might not be sufficient for multisegment fusion procedures.6
Although BMP-2 use eliminates autograft donor-site morbidity various complications depending on the surgical site have been described and led to an FDA warning letter issued in 2008.7,8 The widespread use of BMP-2 was further tempered after the results of the Yale Open Data Access (YODA) project were published.13 The authors concluded that in spinal fusion the use of BMP-2 has no proven clinical advantage over bone graft and could be associated with important complications.14
Among these complications vertebral osteolysis following BMP-2 use has been reported, as described in this case report.15-17 Although the definitive cause for the development of vertebral osteolysis is still unclear it is assumed that the osseos remodeling potential of BMP-2 may lead to bone resorption observed within the vertebral body.17 This adverse effect may be influenced by other factors including endplate preservation, carrier and dose of rhBMP-2 as well as the positioning of the interbody graft.
Osteolysis and aggressive remodeling resulting in loss of construct stability have been reported in studies examining certain stand-alone procedures as well. Additional construct protection with posterior pedicle screws had a positive effect on the fusion outcome. This indicates that stability may also be important for the clinical outcome of the bone remodeling process. Nonetheless it is still unclear if osteolysis is integral to the remodeling process preceding the formation of fusion.10
The role of BMP-2 in inducing osteolysis in the setting of LLIF has not fully been elucidated. We present 3 cases of rapid vertebral osteolysis after utilization of BMP-2 to build on already reported adverse reactions to the biologic. This case report focuses on the adverse effect of osteolytic destruction as a potential complication with BMP-2 utilization. The risks and benefits of BMP-2 utilization as an ICBG alternative must be carefully weighed. Additional studies need to be done to further assess complications and possible long-term outcomes following the utilization of this agent.
The authors certify that they have no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
None.

©2016 Salzmann, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.