MOJ
eISSN: 2374-6939


Case Report Volume 15 Issue 3
1Otologist, Clínica de otorrinolaringología de Antioquia, Columbia
2Otologist, Hospital Pablo Tobón Uribe y Clínica de otorrinolaringología de Antioquia, Columbia
3Otorhinolaryngologist, Hospital Pablo Tobón Uribe y Clínica de otorrinolaringología de Antioquia, Columbia
Correspondence: Adriana Isaza-Marín, Otorhinolaryngologist, Hospital Pablo Tobón Uribe y Clínica de otorrinolaringología de Antioquia, Columbia
Received: May 05, 2023 | Published: May 26, 2023
Citation: Ferrer-Marulanda E, Urquijo DP, Isaza-Marín A. Primary paraganglioma of the facial nerve: a diagnostic challenge. MOJ Orthop Rheumatol. 2023;15(3):99-102. DOI: 10.15406/mojor.2023.15.00626
Paragangliomas are benign tumors of gradual growth that arise from the paraganglion system. They consist of cellular assemblies associated with vascular and neuronal adventitia throughout the body. Moreover, tympanojugular and tympanic PGs are the most common in the head and neck. Primary paraganglioma arising from the facial nerve canal (FN) is rare and challenging to be surgically removed as it is a highly vascularized tumor. Therefore, a preoperative differential diagnosis of an FN tumor is essential for adequate surgical and functional management
Keywords: paraganglioma, facial nerve, temporal bone, facial paralysis
Paragangliomas (PG) are benign tumors of gradual growth that arise from the paraganglion system. They consist of cellular assemblies associated with vascular and neuronal adventitia throughout the body. Moreover, tympanojugular and tympanic PGs are the most common in the head and neck.1–4
About 2-5 % of tympanojugular PGs are malignant, sometimes invading the facial nerve in its descending portion. However, primary paraganglioma arising from the facial nerve canal (FN) is rare and challenging to be surgically removed as it is a highly vascularized tumor. Therefore, a preoperative differential diagnosis of an FN tumor is essential for adequate surgical and functional management.2,3 Furthermore, a case of a primary paraganglioma of the facial nerve canal is presented, describing an infrequent location, scarcely reported, apparently associated with paraganglia, rarely found in the connection between Arnold's nerve and the vertical portion of the FN or within the fallopian canal.
A 61-year-old woman was evaluated by otology for right peripheral facial paralysis with a six months evolution period, initially managed with steroids and Acyclovir without therapeutic response. Physical examination revealed facial motility House-Brackman VI/VI of the right side and I/VI of the left side, an erythematous lesion covered by skin in the right posterior inferior region of the external auditory canal (EAC), and bright tympanic membrane without fluid in the middle ear. In addition, the hearing tests showed a positive bilateral Rinne, and a Weber centered with tuning forks of 512 -256 Hz. Moreover, a simple CT scan of the ear showed attenuation and bony erosion of the stylomastoid foramen, mastoid portion of the facial nerve, and right EAC (Figure 1 & 2).
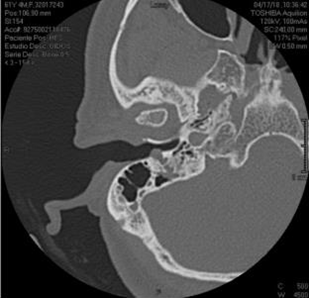
Figure 1 CT of an axial slice of a single ear in the bony canal. Lesion involving the right stylomastoid foramen, the mastoid portion of the facial nerve, and EAC, eroding its posterior wall.
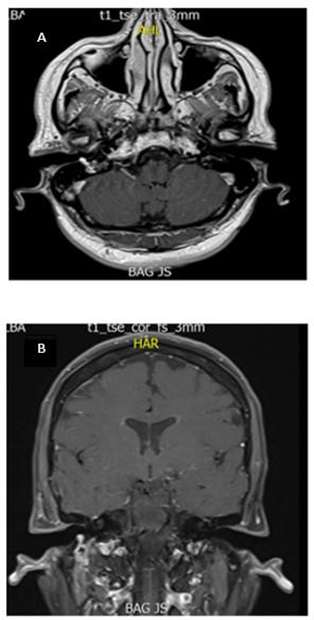
Figure 2 A-B. Axial slice of MRI T1- contrast sequence: Hyperintense lesion involving part of the right EAC. B. Coronal slice of contrasted MR T1 sequence: Hyperintense lesion involving the stylomastoid foramen and right facial nerve.
Magnetic resonance imaging of the brain and neck showed a hypervascular lesion, hyperintense in all sequences involving the stylomastoid foramen and part of the right EAC. Transcanal biopsy showed multiple thin-walled vascular channels with a hemangiopericytoma pattern and crowded cells with scant cytoplasm and ovoid nuclei in the periphery. Immunohistochemical studies were positive for chromogranin and synaptophysin for the diagnosis of paraganglioma. Next, the patient was taken to surgery for resection of the tumor via retro auricular approach with extension to the cervical region and simple mastoidectomy with posterior tympanotomy, with resection of the mastoid tip. Moreover, there was intrasurgical evidence of a lesion infiltrating the FN, originating from the second elbow to the bifurcation in the deep lobe of the parotid, involving the EAC.
Subsequently, decompression of the horizontal and descending FN was performed, the tumor that was firmly adhered to the FN was resected, repair of the FN with interposition of a 5 cm graft of the greater auricular nerve was achieved, and the posterior wall of the EAC was reconstructed with cartilage, fascia, and bone wax graft.
Two months after surgery, there was an improvement in the muscle strength of the middle third of the face. However, eight months later, the patient did not improve facial motility. Subsequently, electromyography was performed, showing denervation of the right FN with no signs of reinnervation. Currently, the patient is in rehabilitation.
Head and neck PGs may have different origins. For instance, the tympanojugular PG arises from the adventitia of the bulb or along Arnold's or Jacobson's nerve, in addition to the tympanic PG.1,2,4 These origins were explained by Guild'y Young et al. in 1941, describing the location of glomus bodies in 88 temporal bones, finding them along the course of the inferior tympanic branch of the glossopharyngeal nerve (Jacobson's nerve) and the auricular branch of the vagus nerve (Arnold's nerve).5–9 Approximately half of the tympanojugular paraganglia are in the adventitia of the anterolateral region of the jugular bulb, near Jacobson's nerve, within the inferior tympanic canaliculus or over the cochlear promontory. The remaining paraganglia lie along the course of Arnold's nerve in the mastoid canaliculus. Although PGs at these sites occasionally invade the facial nerve, Jackson et al. reported a 20% incidence of such extension, and Spector et al. first described extension patterns for glomus tumors through the facial recess and the sinus tympani cells. However, primary PG arising from the FN canal is rare and appears to be explained by paraganglia, which may rarely be found at the connection between Arnold's nerve and the vertical segment of the facial nerve or within the fallopian canal itself. Also, Guild SR observed glomus body cells in this location in only 5 of 88 temporal bones.10,11–19
In this case, the main symptom and sign was paralysis that did not improve with systemic steroid management. Similar to previous reports, the main symptom was facial weakness or paralysis, occasional pulsatile tinnitus, and ear fullness. Two reports showed no facial paralysis (Table 1).3,4,10,15,18,20–23
Most studies found a mass in the EAC during the physical examination (Table 1). Similarly, our patient showed a skin-covered erythematous lesion at the posterior inferior region of the EAC. Commonly, cases, including ours, radiologically show involvement of the tympanic portion and the stylomastoid foramen by a hypervascular tumor that only once has shown a typical salt and pepper image, probably because of their small size and the poor visualization of the slow flow areas and the intramural vessels.3,4,10,15,18,21–26
|
Reference |
Patient detail |
Clinical Presentation |
Image |
Location and extent |
Treatment |
|
Bartels et al.15 |
Woman 30 years old |
Six months of facial paralysis, aural fullness, and retrotympanic mass. |
CT scan |
N. descending canal |
Facial nerve surgery and grafting |
|
Bartels et al.15 |
Male 40 years old |
Two years of facial paralysis, pulsatile tinnitus, conductive hypoacusis, and retrotympanic mass. |
CT scan |
N. descending facial canal With soft tissue retrotympanic |
Facial nerve surgery and grafting |
|
Dutcher and Brackman et al.22 |
Woman 50 years old |
Five months of facial paralysis |
CT scan |
N. descending facial canal |
Facial nerve surgery and grafting |
|
Kania et al.18 |
Woman 63 years old |
Nine months of facial paralysis, pulsatile tinnitus, otalgia |
CT/MRI |
N. descending facial canal and distal horizontal portion With soft tissue retrotympanic |
Facial nerve surgery and grafting |
|
Petrus et al.10 |
Woman 74 years old |
Five years of facial paralysis, retrotympanic mass |
CT scan |
Descending facial nerve canal Extension to the mastoid styloid foramen and EAC |
Biopsy and radiotherapy |
|
Petrus et al.10 |
Woman 74 years old |
Pulsatile tinnitus |
CT scan |
N. descending facial canal and extension to EAC |
Biopsy and radiotherapy |
|
Connor et al.21 |
Woman 55 years old |
Six months of facial paralysis, parotid mass, otalgia |
CT /US |
Descending facial nerve canal Parotid extension |
Facial nerve surgery and grafting |
|
Waldron et al.23 |
Male 71 years old |
Pulsatile tinnitus, retrotympanic mass |
CT scan |
Descending facial nerve canal |
Treatment not described |
|
Takahashi et al.3 |
Male 23 years old |
Eight months of facial paralysis |
CT scan/MRI |
Facial nerve from the second elbow to the stylomastoid foramen, MRI showed heterogeneous T1 and T2 iso- intensity. |
Facial nerve surgery and grafting |
|
Ferrer et al. |
Woman 61 years old |
Six months of Facial paralysis, retrotympanic mass |
CT/MRI |
Descending facial nerve canal Extension to the stylomastoid foramen and EAC. MRI hypervascular lesion, hyperintense in all sequences involving the stylomastoid foramen and part of the EAC. |
Facial nerve surgery and grafting |
Table 1 Clinical presentation, imaging, location/extent, and treatment of confirmed facial glomus
CT, computed axial tomography; MRI, magnetic resonance imaging US, ultrasonography; N, facial nerve; EAC, external auditory canal
Histologically, the evaluated tumor showed a tissue constituted by multiple thin-walled vascular channels, which branched, showing a hemangiopericytoma pattern, the cells were crowded in the periphery, with scanty cytoplasm and ovoid nuclei were consistent with the typical characteristics of described PGs. In addition, demarcated lesions were highly vascularized and formed by cellular nests (Zellballen) separated by thin conjunctival septa. Remarkably, as these tumors are derived from the paraganglia, they consist of two cell types: principal cells or type I cells with cytoplasmic granules containing catecholamines, positive for stains such as neuronal specific enolase, chromogranin A, synaptophysin, serotonin and negative for Carcinoembryonic antigen, Calcitonin, Muscle markers, and Glial fibrillary acidic protein and constituted by Schwannlike satellite cells, or type II cells positive for stains such as S100 fibrillary and Glial fibrillary acidic protein.1,3,4,10, 21
In this case, immunohistochemistry analysis included the following stains: SOX10, STAT6, CK.CD56, CD99, BCL2, CD45, synaptophysin, and chromogranin. Moreover, the last two were positive, confirming the diagnosis of paraganglioma.
Surgical excision is the treatment of choice that should be performed according to the involvement of the FN. For example, when PG induces paralysis secondary to compression with epineural involvement, microdissection of the tumor from the NF, rather than sacrifice, would be considered the appropriate therapeutic option. Likewise, a successful postoperative facial function can only be obtained by preserving the anatomical and functional integrity of the nerve. In contrast, massive FN infiltration, as in this case, requires a complete excision of the involved and reconstructed facial nerve segment to ensure at least facial tone.1–4,10,15,18,21,22,23
The primary PG of the facial nerve is a rare tumor. This report is the only one made in Colombia and leads to practice staged diagnosis to prevent mistakes when making decisions in treatment and rehabilitation since its surgical removal is much more complex, considering it is a vascularized tumor, unlike other more common tumors of the FN.
None.
The authors declare no conflicts of interest.

©2023 Ferrer-Marulanda, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.