MOJ
eISSN: 2374-6939


Review Article Volume 6 Issue 2
1Radiology Department, Cairo University, Egypt
2Rheumatology and Rehabilitation Department, Cairo University, Egypt
3Nephrology department, Cairo, Egypt
4Department of Psychology health and technology, University of Twente, Netherlands
Correspondence: Rgaba Y, Radiology Department, Faculty of Medicine, Cairo University, Egypt
Received: November 02, 2016 | Published: November 2, 2016
Citation: Rgaba Y, Emad Y, Gheita T, Hawaas M, Rasker JJ (2016) Differentiation of Osteoporotic and Neoplastic Vertebral Fractures by Chemical Shift {In-Phase and Out-of Phase} Magnetic Resonance Imaging and Diffusion Weighted Sequence. MOJ Orthop Rheumatol 6(2): 00217. DOI: 10.15406/mojor.2016.06.00217
In elderly population neoplastic wedging of the spine may be an early manifestation of underlying malignancy and differentiating benign wedging due to underlying osteoporosis from malignant wedging has been an important and sought after goal of imaging. Other challenging clinical case scenario is the occurrence of benign osteoporotic vertebral collapse in a patient already known to have underlying malignancy .Although magnetic resonance imaging (MRI) is a sensitive method for assessing bone marrow, it lacks specificity. The problem is that abnormal signal intensity in benign compression fractures on conventional MR imaging can be similar to that seen in vertebrae with underlying malignancy. Although certain morphologic signs may be helpful for assessing the cause of the fracture yet these lack specificity. The presence of both fat and water in normal marrow results in suppression of signal intensity on the opposed-phase images. In benign osteoporotic collapse, no marrow replacement has occurred, thus the existence of normal marrow fat should result in suppression of signal intensity on the opposed-phase images; while in malignant collapse the normal fat-containing marrow is replaced with tumoral process which should result in lack of suppression on the opposed phase images. Diffusion-weighted sequences are sensitive to molecular motion because random motion of water molecules in gradient fields produces phase dispersion and, therefore, signal attenuation. The current review will focus on the advent of both modalities and the value to order them in proper clinical setting.
Keywords: Osteoporotic vertebral fracture; Neoplastic vertebral fractures;Chemical shift (in-phase and out-of phase) MR imaging; Diffusion weighted sequence
A Medline/Embase search was undertaken from 1980 to July 2016 using the following key words; Osteoporotic vertebral fracture; neoplastic vertebral fractures; chemical shift {in-phase and out-of phase} MR imaging; diffusion weighted sequence and combinations thereof. Only articles related to chemical shift MRI and diffusion weighted MR imaging of the spine were recruited. Details of all the studies were described in Table 1.
|
First Author [ref.] |
Year |
Category of Bone Marrow Evaluation |
Technique |
|
Ragab et al. 7 |
2009 |
Osteoporotic and neoplastic |
Chemical shift MRI |
|
Baker et al.11 |
1990 |
Benign versus pathologic |
MRI , Chemical shift MRI |
|
Wismer et al.14 |
1985 |
Bone marrow |
Chemical shift MRI |
|
Zajick Jr et al.15 |
2005 |
Benign and malignant bone marrow |
chemical shift MRI |
|
Eito et al.16 |
2004 |
Malignant bone marrow |
chemical shift MRI |
|
Erly et al.17 |
2006 |
Malignant and acute benign compression |
chemical shift MRI |
|
Zampa et al.18 |
2002 |
Benign and malignant vertebral lesions |
chemical shift MRI |
|
Nakagawa et al.19 |
2000 |
Benign and malignant vertebral lesions |
Diffusion-weighted sequence |
|
Lang P et al.20 |
1998 |
Osteogenic sarcoma |
Diffusion-weighted sequence |
|
Spuentrup E et al.21 |
2001 |
Benign fracture edema and tumor infiltration of the vertebral body |
Diffusion-weighted sequence |
|
Baur A et al. 22 |
2001 |
Osteoporotic fractures and pathologic compression fractures |
Diffusion-weighted sequence |
|
Hackländer T et al.24 |
2006 |
Vertebral metastases due to prostate cancer versus other primary tumors |
Diffusion-weighted sequence |
|
Byun WM et al.25 |
2002 |
Metastatic disease of the spine |
Diffusion-weighted sequence |
|
Lasbleiz J et al.26 |
2006 |
Spine tumors |
Diffusion-weightedsequence |
|
Del Vescovo et al.31 |
2014 |
Metastatic disease of the spine |
Diffusion-weighted sequence |
|
Geith T et al. 29 |
2015 |
Benign and malignant vertebral lesions |
Diffusion-weighted sequence |
|
Park HJ et al. 30 |
2016 |
Benign and malignant vertebral lesions |
Diffusion-weighted sequence |
Table 1 ;Source material for review: articles dealing with MRI, chemical shift MRI, diffusion weighted sequence in different clinical settings by categories whether normal bone marrow appearance, osteoporotic wedging or neoplastic wedging.
Normal bone marrow and its MRI features
The bone marrow is the 5th largest organ of the human body. Its main function is hematopoietic, to provide the optimal supply of circulating platelets, white and red blood cells to meet the body’s requirements for coagulation, immunity, and oxygenation. Hematopoietically active bone marrow is referred to as hematopoietic marrow or red marrow. Red marrow contains approximately 40% water, 40% fat, and 20% protein.
Hematopoietically inactive marrow is referred to as yellow marrow or fatty marrow. It contains approximately 15% water, 80% fat, and 5% protein. These differences in chemical composition account for the appearance of red and yellow marrow on various MRI pulse sequences. There is also a structural difference between red and yellow marrow. In particular, the vascular network of red marrow can be characterized as being rich, while that of yellow marrow is sparser. Normal hematopoietic marrow in the axial skeleton also has fat and water components (red marrow has about 40% fat content, while yellow marrow has 80% fat content).1
There is a predictable pattern of bone marrow changes from infancy to adulthood.2 Red marrow or hematopoietically active marrow is gradually replaced by yellow marrow or hematopoietically inactive marrow over time.3 Red marrow conversion begins at the central diaphyses and expands outward within long bones. Additionally, marrow conversion begins in the epiphyses simultaneously with ossification. At birth, all marrow is hematopoietically active.3-4
In children, the normal bone marrow is highly cellular, which leads to a low signal intensity on plain T1-weighted images and high signal intensity on STIR- or fat saturated T2-weighted magentic resosnance (MR) images. With increasing age, a conversion from this highly cellular marrow in children to fatty marrow in adults occurs with a gradual increase of the bone marrow signal on T1- weighted MR images and a gradual decline of the bone marrow signal on STIR- or fat saturated T2-weighted MR images over time. This conversion also follows a particular distribution pattern within the skeleton which starts in the peripheral skeleton and progresses centrally. Within long bones, it first involves the epiphyses, then the diaphyses and, finally, the metaphyses. In the vertebrae, it starts in the center, around the venous plexus, and progresses peripherally. In adults, the signal intensity of the normal bone marrow is typically hyperintense to surrounding muscle and intervertebral disks on T1-weighted MR images and hypointense to surrounding muscle on STIR- or fat saturated T2-weighted MR images. The knowledge of this pattern of conversion is useful to differentiate normal cellular marrow from focal or diffuse neoplastic involvement and to recognize treatment effects and tumor recurrence.5
At ages 1-10 years, the majority of the diaphyses of the major long bones as well as the cranium have converted to yellow marrow. At ages 10-20 years, most of the marrow in the limbs is yellow. By the age of 25, the normal adult marrow pattern (consisting of red marrow only in the axial skeleton, sternum, ribs, and proximal femora and humeri) is achieved .Some adults may exhibit reconversion of yellow to red marrow. The appearance of this reconverted marrow is fairly heterogeneous and can lead to misdiagnosis of pathology.4
MRI is the imaging modality of choice for the investigation and assessment of bone marrow disorders. Precise interpretation of MR sequences of bone marrow requires an understanding of the anatomy, physiology, and conversion patterns of bone marrow. Summary of the MRI features of normal bone marrow are summarized in Table 2.6
|
Imaging Technique |
Red Marrow |
Yellow Marrow |
|
T1w |
Mild increase of signal |
High signal |
|
T2w |
Intermediate signal |
High signal |
|
T2 FSE w/ FS |
High signal |
Intermediate to low signal |
|
Out-of-phase GRE |
Low signal |
High signal |
Table 2: MRI appearance of normal bone marrow.
MRI: magnetic resonance imaging; FSE: Fast spin Echo; FS: Fat Supression.
Osteoporotic versus neoplastic wedging of the spine: a diagnostic challenge
Differentiation between acute benign osteoporotic and neoplastic vertebral collapse is a common clinical problem and impose diagnostic challenge for both clinicians and radiologists. This task of differentiation has been an important aim of imaging and challenging as well.
Particularly in the elderly people, a neoplastic fracture may represent the first manifestation of a malignancy and wedging among elderly population with known neoplasm is not a necessarily metastatic wedging and may be due to underlying osteoporotic process among this age group.7 Moreover vertebral fractures may occur even without trauma or after minor trauma.8 Furthermore benign compression fractures of vertebral bodies may pose a diagnostic dilemma in patients with primary neoplasms and suspected skeletal metastases or in elderly patients without a known, but potential, primary tumor.9
Establishing the accurate diagnosis is of great importance in determining treatment, surgical intervention, and prognosis. T1- and T2-weighted sequences without or with fat saturation show similar signal intensities for both benign osteoporotic and neoplastic fractures [Figure 1 & 2]. Thus, the morphology of bone marrow replacement has been evaluated for prediction of the benign or pathologic cause of a fracture. Paravertebral soft-tissue masses [Figure 2] and infiltration of posterior elements [Figure 2] are the most reliable MRI signs of a malignant fracture.10
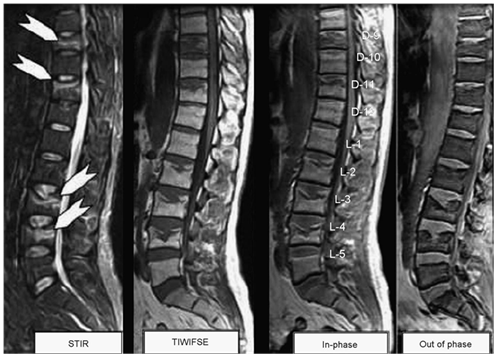
Figure 1 Multiple vertebral benign osteoporotic wedging, STIR sequence shows marrow edema parallel to the end plate (white arrows) of D9, D11, L3 and L4 vertebrae with corresponding enhancement on T1-gadolinium fat sat images. All show significant signal drop on out of phase images consistent with benign collapse.
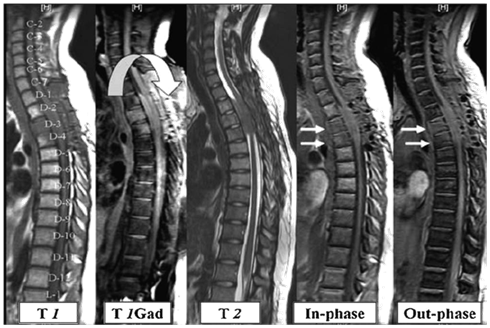
Figure 2 Patient with bronchogenic carcinoma showing multiple neoplastic infiltrative lesions in D12, L1, and L2 vertebrae, they reflect bright signal on STIR sequence with bulging posterior border of L2 (white curved arrow). No significant signals drop on out of phase image relative to in-phase images.
However, the absence of these findings does not always exclude a malignant process. The problem is that abnormal signal intensity in benign compression fractures on conventional MR imaging can be similar to that seen in vertebrae with underlying malignancy. Although certain morphological signs may be helpful for assessing the cause of the fracture yet these lack specificity. Benign fractures usually showing incomplete replacement of bone marrow, while in chronic compression fractures, fat signal intensity was preserved.10
In terms of MR findings, Baker et al.11 observed that acute benign fractures more often show inhomogeneous low signal intensity on T1-weighted spin-echo and fat-suppressed images and inhomogeneous high signal intensity on T2- weighted and STIR images [Figure 1]. In contrast, pathologic fractures showed more homogeneous replacement of the bone marrow, which reflects diffuse bone marrow replacement by tumor cells. Similarly, Yuh et al.12 found that complete loss of signal intensity in the bone marrow on T1-weighted MR images provides a high level of accuracy in diagnosis of neoplastic fractures.
Chemical shift {in-phase and out-of phase} MR imaging
Normal hematopoietic marrow in the axial skeleton also has fat and water components (red marrow has about 40% fat content, while yellow marrow has 80% fat content).13 Early in the development of clinical MR imaging systems, it was suggested that proton chemical shift MR imaging would be a valuable role to standard MR imaging techniques for the study of bone marrow in vivo.14
Marrow infiltrative processes, such as malignant neoplasms, tend to replace the fatty marrow components completely. Accordingly, researchers have hypothesized that chemical shift MR imaging might be a useful technique for the evaluation of axial bone marrow lesions. Chemical shift MR imaging can demonstrate the relationship between the amount of fat and water that coexist in the same voxel. Osseous elements, however, will also affect this relationship; thus, the degree of signal intensity change may not be proportional to the quantity of hematopoietic marrow alone. Hemangiomas for example are slowly growing, benign neoplasms that are commonly found in the vertebral bodies. Histopathologically, they consist of thin-walled, blood-filled vessels and sinuses that are lined by endothelium, are interspersed among the bone trabeculae, and have a variable amount of fat. Some, such as those with predominant fat content, do not demonstrate a drop in signal intensity because there are few or no nonlipid elements. However atypical hemangiomas [Figure 3], which contain only small or microscopic quantities of fat, may demonstrate the utility of chemical shift MR imaging because they will lose signal intensity on out-of-phase images.15
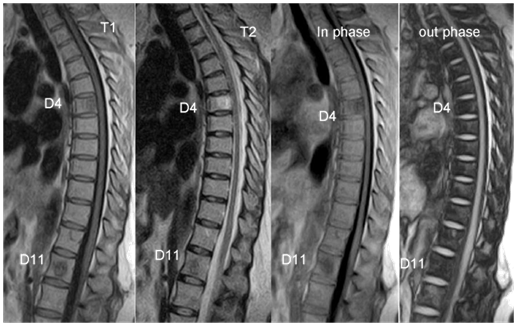
Figure 3 Two vertebral hemangiomas at D4, D11 levels (white arrows), the appearance showed signal drop (D4) on out-phase images.
Recently, Eito et al.16 compared the signal intensity ratios (SIRs) of normal and neoplastic compression-fractured vertebrae in 108 patients by means of dual-phase chemical shift MRI. Their study included three groups; group 1: normal vertebrae (N= 30); group 2: non-neoplastic compression fractured vertebrae (N= 58); and group 3: neoplastic compression-fractured vertebrae (N= 20). The mean SIRs of the three groups appeared to be significantly different. They concluded that opposed-phase and in-phase gradient-echo MR imaging of vertebral signal intensity abnormalities can help to predict the nature of compression fractures.
In another study Erly et al.17 used the same technique to differentiate between benign and malignant fractures of the spine. The authors evaluated a total of 25 consecutive patients with suspected malignancy [lymphoma N= 4, breast cancer N= 3, multiple myeloma N= 2, melanoma N= 2, prostate N= 2, and renal cell carcinoma N= 1] for trauma to the thoracic or lumbar spine. Areas that were of abnormal signal intensity on the T1 and T2 sequences were identified on the in-phase/opposed-phase sequences. The authors found a significant difference (P < .001) in the mean SIR for the benign lesions compared with the malignant lesions. If a SIR of 0.80 as a cutoff is chosen, with >0.8 defined as malignant lesions and <0.8 defined as a benign lesions, in-phase/opposed-phase imaging correctly identified 19 of 20 malignant lesions and 26 of 29 benign lesions (sensitivity, 0.95; specificity, 0.89).
In another work Zampa et al.18 evaluated 86 vertebral lesions by using an MR protocol consisting of a T1-weighted spin echo and an out of- phase gradient-recalled echo MR imaging sequence. They observed that a cut-off value of 1.2 resulted in 88.8% sensitivity, 80.5% specificity, 84.9% accuracy, 86.4% negative predictive value, and 83.3% positive predictive value. They concluded that marrow lesions with values that were higher than the cut-off value were considered neoplastic, whereas other lesions with values that were lower than the cut-off value were considered benign. Zajick et al.15 retrospectively assessed the use of chemical shift magnetic resonance (MR) imaging technique in differentiating benign from malignant marrow abnormalities. The authors examined a total of 569 normal vertebrae in 75 patients (42 women, 33 men; mean age, 57.5 years; age range, 26-84 years) (control group) and 221 lesions in 92 patients (50 women, 42 men; mean age, 59.0 years; age range, 27-85 years) (study group) who had focal vertebral marrow abnormalities by using 1.5-T chemical shift MR imaging. The authors found a substantial decrease in signal intensity for all normal vertebrae and for benign lesions.
Ragab et al. 7 established the cut-off value of the signal intensity drop on chemical shift (MRI) to obtain appropriate sensitivity, specificity and cut off value to differentiate osteoporotic from neoplastic wedging of the spine. In their study a total of 40 patients were recruited, 20 with osteoporotic wedging [Figure1] and 20 neoplastic [Figure 2 & 3]. In their study the authors observed a substantial reduction in signal intensity in all lesions in both groups. The proportional changes observed in signal intensity of bone marrow lesions on in-phase compared with out-of-phase images showed significant differences in both groups (P < 0.05). At a cut-off value of 35%, the observed sensitivity of out-of-phase images was 95%, specificity was 100%, positive predictive value was 100% and negative predictive value was 95.2%. In contrast to the study by Ragab et al.7 Zajick et al.15 suggested that a decrease in signal intensity greater than 20% on out-of phase images compared with in-phase images should be used as a cut-off threshold for normality to allow distinction between benign and neoplastic vertebral marrow lesions.
The discrepancy in the cut off value obtained in the study by Ragab et al.7 compared to Zajick et al.15 may be explained by the difference in the type of lesions. In the study by Zajick et al.15 the authors evaluated different lesions like endplate degeneration, Schmorl's nodes with edema, hemangiomas, benign fractures and different patterns of metastatic lesions which stratified in their study into three different categories: lytic, blastic, or mixed. While Ragab et al.7 only compared only established osteoporotic and neoplastic wedging. It should be noted that bone marrow lesions in the vertebral bodies due to different pathologies may displays somewhat variable behavior at chemical shift MR imaging. Nevertheless, Zajick et al.15 observed that 8(16%) of 51 lesions demonstrated a decrease in signal intensity that was greater than 20%. Of the lesions that demonstrated false-negative findings at chemical shift MR imaging, four had a decrease in signal intensity that was greater than 40%, with the greatest decrease in signal intensity being 75.9%. The authors stated that this discrepancy may be in part related to the lesion subtype. Lytic lesions loose less signal intensity than blastic lesions. The substantial number of metastases that could not be characterized (unknown lesions) in their study also contributed to the variable behavior of malignant lesions at chemical shift MR imaging. Given that the type of the lesion itself may lead to different behaviors on chemical shift MRI
The fluid sign
The fluid sign which is defined as a focal, linear, or triangular area of strong hyperintensity on STIR images on a background of diffuse hyperintensity in the vertebral body [Figure 4] because of acute collapse may be regarded as an additional morphologic feature that supports the benign osteoporotic nature of an acute vertebral fracture. Although this finding is significant, a tumor cannot be excluded because of this sign only.9
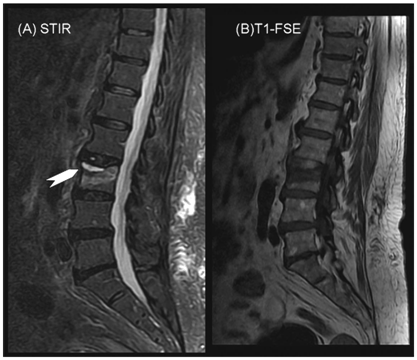
Figure 4 STIR sequence showing linear bright signal at the upper end plate of L3 (fluid sign) with a back ground of mild hyper-intensity.
Figure 4B T1WI showing hypo-intensity parallel to the upper end plate of L3.
The fluid sign at MR imaging may be regarded as an additional morphologic feature that supports the benign osteoporotic nature of an acute fracture. Although this finding is significant, a tumor cannot be excluded because of this sign. Other morphologic features or diffusion-weighted imaging should be considered if the diagnostic decision is difficult.
The fluid sign at MR imaging may be regarded as an additional morphologic feature that supports the benign osteoporotic nature of an acute fracture. Although this finding is significant, a tumor cannot be excluded because of this sign. Other morphologic features or diffusion-weighted imaging should be considered if the diagnostic decision is difficult.
Ragab et al.7 observed that fluid sign was more frequently present among patients with osteoporotic collapse (13/20) compared to those with neoplastic wedging (2/20). The findings of Ragab et al.7 are consistent with and extend those observed by Baur et al.9 who evaluated the occurrence, location and shape of the fluid sign in acute osteoporotic and neoplastic vertebral compression fractures at magnetic resonance (MR) imaging in 87 consecutive patients with acute vertebral compression fractures due to osteoporotic (N= 52) or neoplastic (N= 35) infiltration. They found that the fluid sign was significantly associated with osteoporotic fractures (P < 0.001). They concluded that the fluid sign is featured in acute vertebral wedge fractures that show bone marrow edema (BME). It can be an additional sign of osteoporosis and rarely occurs in metastatic fractures.
Diffusion-weighted sequences
Vertebral fracture in an older person, particularly one with a history of cancer, is due to OP or is the result of a metastasis, is a not infrequent clinical problem that has important prognostic and therapeutic implications. The two types of fracture are usually indistinguishable on plain radiographs and require higher order imaging for diagnosis. Complete replacement of the normal bone marrow and a convex posterior contour of the vertebral body, favors a fracture of neoplastic origin. Using diffusion-weighted imaging and/or chemical shift imaging may be helpful in difficult cases.7,19 Diffusion-weighted sequences have been proposed as a helpful adjunct in the differentiation of acute benign osteoporotic fractures from pathologic fractures of the spine.20 Diffusion-weighted sequences are sensitive to molecular motion because random motion of water molecules in gradient fields produces phase dispersion and therefore, signal attenuation. Water in vital tumor cells shows lower mobility as a result of cellular structures.21 In the presence of diffusion-sensitizing gradients, this finding should result in a lower signal attenuation compared with stronger dephasing of more mobile extracellular water with extensive signal attenuation. Accordingly, diffusion weighted imaging could differentiate benign (osteoporotic and traumatic) and malignant vertebral fractures.22 Hypo- or isointense signal intensity in an acute vertebral wedge fracture indicates a benign osteoporotic fracture [Figure 5], whereas hyperintensity indicates a metastatic fracture [Figure 6]. Hypo- or isointense signal was diagnostic for an acute benign fracture, whereas high signal intensity was suggestive of pathologic bone marrow replacement. The low signal intensity of acute benign vertebral compression fractures on diffusion-weighted images may be due to bone marrow edema, leading to an increase in the mean free path length of water protons and therefore to a signal loss in the fractured areas. Moreover increasing diffusion weighting can reduce false-positive hyperintense osteoporotic fractures or make hypointensity more obvious in cases of osteoporotic fractures. It is noteworthy to mention that if the findings on routine T1-weighted SE and STIR images are not completely conclusive for a diagnosis of acute benign or pathologic vertebral compression fracture, then diffusion-weighted imaging of the spine is indicated.23
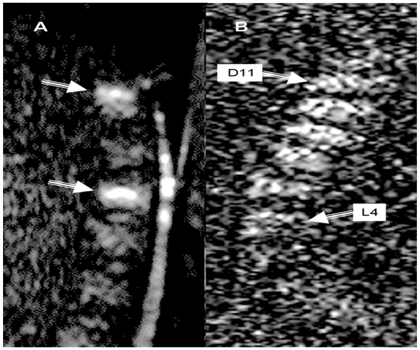
Figure 6 Metastatic breast cancer.
Figure 6B multiple myeloma (D11-L4), Sagital diffusion weighted images (DWIs) both lesions showed evidence of diffusion restriction indicated by white arrows.
Given that, the appearances of infiltrative marrow disease are explained on the basis of marrow composition and whether disease causes proliferation, replacement, or even depletion of the normal marrow components.24 Therefore, metastases are represented in diffusion-weighted images by increased signal intensity in comparison to unaffected vertebrae.25 In vertebral metastatic lesions due to prostate cancer the situation may differ. In general, the metastatically affected vertebrae appear hyperintense in the diffusion-weighted images. This observation is only true for some vertebral metastases due to prostate cancer. The cause for this seems to be the degree of sclerosis of the metastases. Thus, it cannot be generally deduced from the hypointensity in diffusion-weighted images that a lesion is benign.25
Most important is that diffusion weighed sequence can aid monitoring response the therapy in case of neoplastic wedging. It has been observed that with successful therapy diffusion-weighted MR imaging showed decreased signal intensity of metastatic disease of the vertebral bone marrow.26 Finally performing diffusion weighted imaging of the spine is easy with new MR technology, but does not serve as a substitute for the routine MRI sequences. In the future, it could become an important diagnostic tool in this domain.27 Important to note is that ADC values of the lumbar spine demonstrated gender- and age-related differences. These findings likely reflect changes in the cellular component of the lumbar bone marrow.28 In recent work Geith et al.29 tested the hypothesis that ADC in vertebral bone marrow of benign and malignant fractures is related to the volume of the interstitial space, as determined with dynamic contrast-enhanced (DCE) MR imaging. Their results supported the hypothesis that the ADC of a lesion is inversely correlated to its cellularity. This could explains the previous observations that ADC is reduced in more malignant lesions.30 Whole-body DW-MR imaging was now considered more sensitive in the detection of osseous metastases than were skeletal scintigraphy and CT bone scanning.31
Nevertheless in postmenopausal women with OP exhibited a corresponding increase in vertebral marrow fat content as the bone density is decreased. Marrow fat content and ADC were related to the bone density. MRS and DWI are helpful in evaluating the bone marrow changes in postmenopausal women.32
Chemical shift MRI imaging is a useful technique for evaluating patients with vertebral collapse, whether due to underlying osteoporosis or neoplastic process. More recently Diffusion-weighted sequences appear to be more sensitive technique for task of differentiation. The exact sensitivity, specificity, positive and negative predictive values of both techniques needs further evaluation in more studies. The advent of new MRI technology will enable clinicians to solve previously unsolved issues related to the task of differentiation between ostoporotic and neoplastic wedging of the spine and undoughtly will allow for early and proper comprehensive approach for early management of both conditions.
None.
None.

©2016 Rgaba, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.