MOJ
eISSN: 2374-6939


Case Series Volume 11 Issue 5
1Pediatric Rheumatology, Tripoli Children’s Hospital, Libya
2Pathology, Tripoli Faculty of Medicine, Libya
3Adult Rheumatology, Dr. Fakhry Hospital, Saudi Arabia
4Community Medicine, Tripoli Faculty of Medicine, Libya
5Dermatology, Central Tripoli Hospital, Libya
Correspondence: Awatif M Abushhaiwia, Pediatric Rheumatology, Tripoli Children’s Hospital, Libya, Tel 00927880360
Received: October 10, 2019 | Published: October 31, 2019
Citation: Abushhaiwia AM, Abushhiwa NB, Kamel M, et al. Childhood pyoderma gangrenosum: diagnostic challenges and management of two cases from Libya. MOJ Orthop Rheumatol. 2019;11(5):188-193. DOI: 10.15406/mojor.2019.11.00499
Background: Pyoderma gangrenousm (PG) is an idiopathic ulcerative, non-infective chronic inflammatory skin disorder of unknown etiology. The most common reported underlying diseases in pediatrics are inflammatory bowel disease, followed by hematologic disorders, vasculitis, immune deficiencies and pyogenic arthritis, pyoderma gangrenosum and acne (PAPA) syndrome. More than half of the cases occur with no underlying disease. The most frequently treatment should be tailored according to the underlying etiology. It includes systemic steroids, corticosteroid sparing agents such as dapsone, cyclosporine, azathioprine and currently TNF –alpha inhibitors such as etanercept, adalimumab and infliximab is a promising treatment of refractory PG. Response to treatment is high with cure rates reaching 90%. A high index suspicion and a through workup are mandatory in the management of pediatrics PG.
Objective: To describe the clinical presentation, laboratory tests and challenges with treatment trials in two pediatric cases.
Methods: Clinical information was gathered from the history, which was taken from their parents after an informed consent.
Result: For the first time we report two pediatric cases in Libya who diagnosed with refractory pyoderma gangrenousm with no associated systemic diseases except PAPA which can’t be excluded since no genetic tests available in all public hospitals in Libya, we report PG in two Libyan children of 2 year-old and 5 year-old, they presented with skin lesions beginning as a small pustule that progressed to very painful large ulcer with irregular borders. There were healed ulcers with scaring present on chest, both upper and lower extremities and abdominal wall. Laboratory tests for both cases showed an increase in white blood cell counts mainly neutrophils, anaemia, increased platelets count, elevated erythrocyte sedimentation rate, and elevated C-reactive protein. No haematological abnormalities were seen in peripheral blood smears except neutrophilic response, the red cell indices were suggestive of iron deficiency anaemia. Cultures for bacteria and fungi from skin lesions, and blood cultures were repeatedly negative. Immunoglobulin assay (IgG, IgM, IgA, IgE) was normal. The antinuclear antibody, cytoplasmic antibody, and antiphospholipid antibodies, tests for hepatitis B, C and HIV were all negative. They didn’t have symptoms suggest ulcerative colitis, their colonoscopy was normal. Histopathology from their skin was consistent with pyoderma gangrenousm. Both cases were moderate response to high dose of oral prednisolone 2mg\kg per day complicated, however by growth retardation and cushingoid habitus and minimal response to corticosteroid sparing agent azathioprine. Anti TNF-alpha inhibitors etanercept, which has been available at hospital since March 2018, we used it in case 2 because he was inadequate response to azathioprine. Both cases showed briefly remitted on azathioprine with relapsed at least 2-3 episodes per year. The ulcer lesions healed with cribriform scaring within 6-8 weeks in each episode.
Conclusion: Pyoderma gangrenousm has recently been included within the spectrum of auto- inflammatory diseases, which are characterized by recurrent episodes of sterile inflammation, without circulating auto antibodies and auto reactive, which can be diagnosed by genetic tests, which is unfortunately not available in public hospitals in Libya. We couldn’t either be able to start other biological treatment because of the civil war crisis in Libya.
Keywords: etiology, cribriform scars, pyogenic arthritis, hyperostosis, bowel disease
Pyoderma gangrenosum is a rare ulcerative inflammatory cutaneous condition of unknown etiology that is uncommon in childhood,1-3 with only 4% of reported cases occurring in individuals younger than 15.4 The initial lesion in PG is commonly a painful hemorrhagic nodule or pustule that rapidly progresses into a large necrotic boggy ulcer that may resolve with cribriform scars.5 The ulcers can affect any part of the body, with the lower extremities being the most common location. About 50% of patients with PG are associated with an underlying systemic disease, including inflammatory bowel disease,6 hematologic malignancies disease,7 and arthritis.8,9 It may occur in the context of classic syndromes like PAPA (pyogenic arthritis, pyoderma gangrenosum and acne) and SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis), as well as in recently named PASH (pyoderma gangrenousm, acne and supportive).
PG thus presents many clinical challenges. It is difficult to diagnose, is frequently misdiagnosed and often requires a work-up for an underlying systemic disease. Successful management of PG typically requires multiple modalities to reduce inflammation and optimize wound healing, in addition to the treatment of any underlying diseases. Prednisone10 and immunosuppressant agents, such as cyclosporine,11 azathioprine,12 methotrexate,13 cyclophosphamide,14 tacrolimus,15 dapsone,16 mycophenolate mofetil,17 and thalidomide,18 have been used. Nevertheless, the exact response rate for these agents has not been identified, and they appear to exhibit considerable clinical variability. Among these agents, cyclosporine and corticosteroids are most commonly used; however, they have been associated with significant side- effects, leading to death in rare cases.1,9,19 Therefore, identifying a safer and possibly a more efficacious treatment modality for PG is desirable.
Recent reports have indicated that recalcitrant PG can be effectively treated with immunomodulating agents that exhibit activity against tumor necrosis factor-α (TNF-α).20 It has seen mainstays of systemic treatment for PG, although increasing evidence supports the use of biologic therapies, such as tumor necrosis factor- inhibitors for refractory cases of PG. Here we review the clinical presentations and pathological of PG in two cases, as well as its associated conditions, diagnostic work-up, and management.
A 4 year-old Libyan boy from west, born from unrelated parents was referred by a dermatologist to the Rheumatology clinic of Tripoli children’s hospital on June 2016 when he was two years old. The patient had a history of multiple progressive maculopapular ulcers lesion over his body since in November 2015. The patient had no remarkable personal or familial antecedents, and was born full term from a non- complicated pregnancy and labor. At the age of 2 year-old he developed small pustules on an erythematosus, edematous base, which rapidly enlarged to form big ulcers lesions on his face, upper and lower extremities, trunk and abdomen. The patient had been treated with a variety of oral, topical and parenteral antibiotics plus oral steroid; prednisolone 1 mg/ kg per day, topical tacrolimus 0.1% ointment and dapsone gel 5% with inadequate response. Skin biopsy showed a dense neutrophil-rich infiltrate that spanned the dermis with few lymphocytes; a histological finding, which are in favor of pyoderma gangrenousm. Stains and cultures for pathogenic organisms were all negative. At initial presentation to the Rheumatology clinic, the patient had numerous well-circumscribed erythematosus papules and pustules of various sizes and stages, involving the face, thighs, abdomen, thorax with well-circumscribed erosive plaques, central crusting and raised erythematosus borders involving bilateral thighs (Figures 1&2).
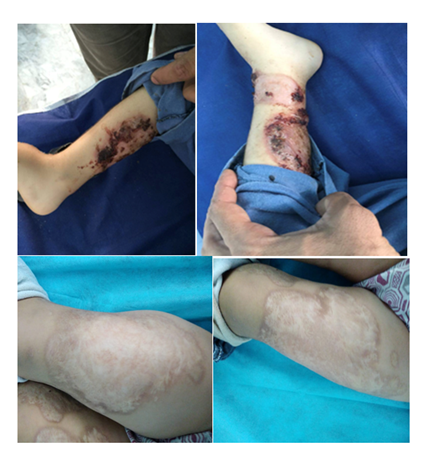
Figure 1 Right leg and left leg upper & lower with ulcerations lesions at presentation, healed with scars on lower extremities (weeks later of steroid therapy azathioprine).
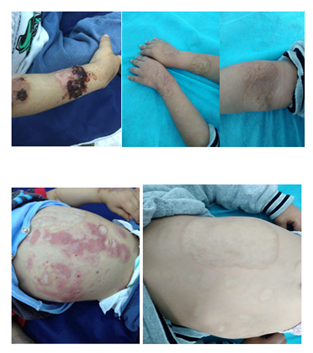
Figure 2 Right arm ulcerations & ulcers showing healed with scaring on both hands, cubital, abdomen and chest. (Weeks later of steroid therapy about lesions on abdomen &trunk).
On clinical examination, the child was febrile with all anthropometric data below the third percentile. All vital parameters were within normal limits. He had erythematosus, pustular, well-demarcated deep ulcers located on the right thigh > left thigh, abdomen, left elbow on cubital fossa (Figure 1A). The leg lesion showed superficial ulceration with red to violet colored erythema, they had gradually increased in size. Theses lesions were culture-negative for bacteria. A punch biopsy was obtained from the lesion on the right leg ulcer. Histological examination revealed neutrophilic dermatose compatible with pyoderma gangrenousm, which was covered with necrotic tissues and healed with cribriform scars (Figure 2B). No evidence of mycobacterial infection. Laboratory examination showed increase white blood cells counts (30000\mm3, with 76.7% neutrophils, 17.6% lymphocytes, anaemia (8.3g\dl hemoglobin), increased platelets (687000\mm3) and elevated ESR 85mm\1hrs, elevated C-reactive protein (164mg\l), No hematological abnormalities were seen in peripheral blood smears with neutrophilic response. hemoglobin electrophoreses to exclude sickle cell anaemia was normal and the red cell indices were suggestive of iron deficiency anaemia, there was no evidence of hematologic malignancies . cultures for bacteria and fungi from skin lesions and blood cultures were repeatedly negative. immunoglobulin assay (IgG, IgM, IgA, IgE) complement levels were normal.
Antinuclear antibody, ASCA, cytoplasmic antinuclear cytoplasmic antibody (c-ANCA), antiphospholipid antibody, hepatitis surface antigen and anti-hepatitis C virus were negative. Enzyme-linked immunosorbent assay for HIV was negative. Liver profile and urine analysis were normal. l. Stool for caloprotectine, colonoscopy and Gastroenterological evaluation revealed no evidence of inflammatory bowel disease two skin biopsy specimens were obtained; histopathology study was consistent with pyoderma gangrenousm.
The patient was treated with oral prednisone 2mg\kg\day, which induced a substantial improved based on the clinical condition and results of the biopsy. Then the PG was refractory to prednisolone 2 mg/kg per day, when new lesions and progression of prior lesions developed Patient was started on topical tacrolimus 0.1% ointment and dapsone 5% gel.
Two days after the initial examination, the patient was admitted to the hospital because of worsening pain and ulcers. The ulcers on his thighs, abdomen was more expanding. We started IV methylprednisolone 30mg/kg for 3 consecutive days, intravenous immunoglobulin 2mg\kg then followed by oral prednisone 2gm\kg\day, the patient had good response after 8 weeks. All skin lesions were healed, then, we tapered oral steroid until 5mg\day, and started azathioprine with dose 1.5mg\kg, increased gradually until 3mg\kg. Over the next two and half years, the patient developed recurrent sterile skin ulcers and PG, there were 2 episodes each years, On 2018 he had only an episode in May 2018, not preceded by trauma, arthritis, or fever, but there was high white blood cells count, anaemia, elevated erythrocyte sedimentation rate and C-reactive protein, Patient was treated with oral prednisone 2mg\kg\day for 8 weeks then tapered until becomes on a dose 3mg\day. The dosage was tapered gradually. 6 months later his condition was under good control he suffered from growth retardation and Cushingnoid habitus. He had moderate response to immunosuppressant treatment of azathioprine.
We thought about PAPA syndrome (pyogenic sterile arthritis, pyoderma gangrenosum [PG] but genetic tests were not available at our hospital. besides we couldn’t start biological treatment because of the civil war in Libya.
A 7 year-old Libyan boy from the south was born from unrelated parent. He was referred to the Rheumatology clinic at Tripoli children’s hospital by a dermatologist on November 2016 for further evaluation. The child presented with skin lesions and scarring over the chest and leg for a period of 6 weeks. He was apparently well a year back, and then developed a pustule over his face then spreads over the neck and upper part of the chest, abdomen and lower extremities.
The lesions ruptured spontaneously forming an ulcer, which was extremely painful. He was admitted to surgical department, where they treated him by intravenous antibiotics for 10 days, local applications, but without any response. Ulcer gradually increased in size and in due course healed with scarring. He had developed more such lesions in various parts of his body associated with high-grade fever that reached to 40-degree cellules. The patient was then admitted again to the dermatological department for 2 weeks as a case of non-healing ulcer. The histopathology examination of his skin lesion was consistent with pyoderma gangrenousm. They treated him with intravenous antibiotics meropenem and vancomycine, oral prednisolone 1mg\kg, intravenous immunoglobulin 2mg\kg for 2 weeks, but there was inadequate response.
The patient developed new lesions on trivial trauma. There was no history of drug intake prior to the onset of his illness. There was an important related Family history when his grandmother from father's side was diagnosed to have a Rheumatoid arthritis. Clinical Examination revealed an anemic child, mild clubbing fingers with all anthropometric data below the third percentile. All vital parameters were within normal limits.
There were large scars present over the face, the chest (Figure 3) abdominal wall and thighs. He had healed ulcers over the left elbow (Figure 4). The ulcers were large with irregular borders and rolled up edges. The base of the ulcer was covered with necrotic material. During his follow up, he developed a small pustule over the dorsal surface of index finger of the right hand (Figure 5). There were no joint swellings.
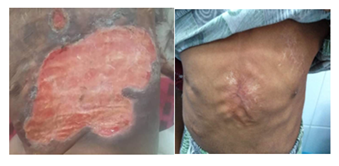
Figure 4 (A) huge ulcer measuring 15X10 cm over his chest and abdomen, (B) lesion resolved after treatment with etenrecpt and high dose oral steroid.
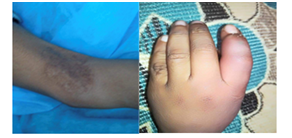
Figure 5 (A) cribriform scar over his left elbow, (B) small pustule lesion over the of dorsal surface of the right index finger.
Systemic examination was unremarkable. Laboratory tests showed increased total white blood cell count 40000/mm3, with 78.8% neutrophils, 17.8% lymphocytes, anaemia (9.5g/dL hemoglobin), increased platelets (410000/mm3). No haematological abnormalities were seen in peripheral blood smears with neutrophilic response, hemoglobin electrophoreses to exclude sickle cell anaemia was normal and the red cell indices were suggestive of iron deficiency anaemia, hematologic evaluation revealed no evidence of hematologic and malignancies, blood cultures were repeatedly negative, bacterial, mycobacterium and fungal cultures were negative and immunoglobulin assay (IgG, IgM, IgA, IgE) complement levels were normal.
Antinuclear antibody, cytoplasmic antinuclear cytoplasmic antibody (c-ANCA), antiphospholipid antibody, hepatitis surface antigen and anti-hepatitis C virus were negative. Enzyme-linked immunosorbent assay for HIV was negative. Liver profile was normal. Urinalysis was normal. Stool for caloprotectine, colonoscopy and Gastroenterological evaluation revealed no evidence of inflammatory bowel disease.
Skin biopsy showed hemorrhagic scale crust with presence of Neutrophils dust, epidermal erosion, focal epidermal ulcer and pseudo epitheliomatous epidermal hyperplasia, the dermis has papillary dermal edema and diffuse inflammatory cell infiltrate composed of mainly neutrophils with karyorrhexis, lymphocyte, few eosinophils and extravasated RBC, the blood vessels has endothelial cell swelling and fibrinoid deposition, smaddy appearance (vasculopathy) the above histological finding was consist with pyoderma gangrenousm .
We started routine local wound care and topical applications. Patient was started on topical tacrolimus 0.1% ointment and dapsone gel 5%. The family was instructed to use non-stock pads with net stocking to dress the active lesions on his huge ulcer on abdomen, right index finger and lower extremities then, prednisolone 2 mg/kg body weight. Since the family and hospital, because shortage of the supplies, in our hospital we were not able to use steroid-sparing drug because of the civil war.
The child showed good response to treatment and within 2months the ulcer on the abdomen started to heal (Figure 4) and the lesion that appeared during his hospital stay did not progress to ulcerations at that time, then the steroid dose was tapered and we added azathioprine 3mg\kg. This combination showed good response. The lesions were healed within 6-8 weeks. In 2018 there were two episodes in January and August. We started with immunomodulating agents that exhibit activity against tumor necrosis factor-etanercept 0.8mg\kg\week subcutaneous adjunctive with oral prednisolone 2mg\kg\day and stopped azathioprine drug the lesions were healed within 8 weeks, however he had severe fungal infection over his face, head and body on December 2018. Etanercept was stopped for 4 weeks due to severe fungal infection and we treated him with local and systemic antifungal treatment; he was improved within 4 weeks (Figure 6). We back him on azathioprine tab 3mg\kg\day. We thought of PAPA syndrome (pyogenic sterile arthritis, pyoderma gangrenousm [PG], and acne but we do not have genetics laboratory access at our hospital, also we couldn’t continue or start another biological treatment because it not available.
PG is rare in children and more in infants younger than 1 year.4 The cause of PG is unknown, but some studies have suggested that abnormal neutrophil chemo taxis is the primary process.5 The initial lesion in PG is commonly a painful hemorrhagic nodules or pustule that rapidly progresses into a large necrotic boggy ulcer that may resolve with cribriform scars.4 The ulcer can affect any part of the body, with the lower extremities being the most common location.
Diagnosing PG in children can be challenging. We reported PG in a two year-old and a five year- old, lesions in their children are also more likely to start as inflammatory pustules and evolve rapidly into ulcerative and necrotic plaques to have involvement of the lower, upper extremities, trunk and abdomen without any associated disorders except PAPA syndrome we couldn’t exclude it because we don’t have genetic laboratory access at our hospital, which was well controlled with a high dose of oral prednisone, azathioprine, etanercept combination with topical application of steroid , tacrolimus or dapsone ointment. As well as ulcers lesion healed with cribriform scaring within six to eight weeks but some episodes healed within 20 weeks. Although our patients showed moderated improvement, we reported the effective use of the combination therapy of topical steroid, tacrolimus 0.1% ointment, dapsone 5% ointment, systemic steroids and immunosuppressant azathioprine in some episodes, which where healed within six to eight weeks without adverse effects if the tacrolimus ointment and dapsone ointment were available at our hospital. Case 2 was considered refractory to chronic systemic steroid, azathioprine, and topical steroids showed little response within 20 weeks, which enabled prednisolone to be discontinued and the prevented of new lesions then decided to start etanercept injection once per week subcutaneous, patient was well tolerated and lesions healed within eight weeks but etanercept was stopped for four weeks due to severe fungal infection (Figure 6). Etanercept is an alternative treatment option for patients with refractory ulcers due to PG1.although our experience is a very limited. Etanercept seems to be tolerated. The most common side effect documented to date is injection site reaction, in rare circumstances; etanercept could be associated with congestive heart failure, pancytopenia, demyelinating disorders, bone marrow suppression and systemic lupus.1
PG in infants has a good response to therapy and healing is usually achieved. This entity in children usually presents with multiple lesions, mainly located on the face, buttocks, thighs and extremities, which, in some instances are associated with pathergy. PG is an inflammatory condition of the skin first described by Brunsting et al.6. PG begins as a pustule or vesiculopustule those progresses to ulcer or deep erosion with violaceous overhanging or undermined borders (Table 1).
Major criteria |
Minor criteria |
Rapid progression of painful necrolytic cutaneous ulcer with an irregular |
History suggestive of pathergy or clinical finding of cribriform scarring. |
Systemic diseases associated with PG. |
|
Histological finding (sterile dermal neutrophilia ±mixed inflammation ± lymphatic vasculitis) |
|
Rapid response to systemic glucocorticoids treatment |
Table 1 Criteria for diagnosis of PG
The histopathological findings in PG are not specific and the biopsy cannot be diagnostic of PG. The importance of biopsy,, is to exclude other diseases. Although vascular inflammation in lesions of PG is not uncommon, the histology is not that of a true vasculitis.8
There is currently no gold standard of treatment or published algorithm for choice of therapy. The majority of data come from case studies that lack a standard protocol not only for treatment administration but also for the objectives.9 Once diagnosed, treatment should be directed first towards the underlying disease that is present (inflammatory bowel disease, especially ulcerative colitis, hematologic malignancy or paraproteinemia, Behcet disease, hepatitis, HIV, systemic lupus erythematosus, PAPA syndrome (pyogenic arthritis, pyoderma gangrenosum, and acne). The diagnosis of PG is, in fact, a diagnosis of exclusion.20 Clinical presentation, along with repeatedly negative cultures showing a non-response to antibiotics, pathergy phenomenon, and a histology showing nonspecific aseptic neutrophilic infiltration, are all suggestive of PG.
Once the diagnosis of pediatric PG is confirmed, the search for an underlying etiology should be undertaken, as pediatric PG can be the initial presentation of a systemic disorder. The common underlying etiology is IBD.21,22 Minute inquiry about gastrointestinal symptoms is crucial, completed by endoscopic evaluation if judged necessary by the clinician the second most common underlying disorder is homeopathies such as myelodysplastic syndrome, leukemia and lymphomas. In such cases, the child commonly presents with general status alteration with hepatosplenomegaly and palpable lymph nodes on physical examination, a complete blood count, blood smear, and bone marrow aspiration are to be done. Pediatric vasculitis can present with PG, with Behcet and Takayusu’s arthritis being the most commonly reported recurrent infections and delayed wound healing suggests immune deficiencies and more specifically, LAD1 deficiency.23 Personal or family history of auto-inflammatory syndromes, pyogenic arthritis, and severe acne\hidradenitis supportive raise the possibility of PAPA, PASH, or PAPASH syndromes. PG a long with arthritis and chronic multifocal recurrent osteomyelitis. In contrast with the previous findings of Graham et al.24 idiopathic PG proved to be the largest subgroup. Thorough history taking, physical exam and workup, combined with a close follow-up, are paramount when faced with PG with no obvious underlying disease.
Treatment strategies include pain control; local wound care, topical applications and systemic drugs. Ulcerative PG is often treated initially with high-dose corticosteroids (prednisone 1–2 mg/kg/d), because of the rapidity of response in 2–3 days. Some ulcers may require months to years to fully resolve other immunomodulators have been used; cyclosporine, can help as a corticosteroid-sparing therapy. However, cyclosporine has its own side-effect profile. Recent data suggest that tumor necrosis factor-alpha–blocking agents may be effective, particularly in refractory cases.25-27 A large randomized controlled study is needed to determine the optimal dosing schedule, durability of the treatment response and long-term risks and benefits for treatment of PG ulcers with etanercept and other biological actions of TNF-α.1
Generalized and ulcerative lesions are the most frequent initial presentation of pediatric PG. The most common underlying etiologies in pediatric PG are IBD, hematologic malignancies, immune deficiencies, vasculitis, and PAPA syndrome. PAPA syndrome should be considered in differential diagnosis in our cases, however we could not exclude it, because genetic testing is not available in public hospitals. In addition idiopathic cases account for 49% of cases. A thorough clinically oriented workup is mandatory to make the correct diagnosis and prescribe the appropriate treatment. Large clinical trials with long follow- up periods are mandatory to determine the exact etiology and prognosis of PG in children. The main concern in is we need to further clarify the role of biological treatment in PG in children.
None.
The authors declare there are no conflicts of interest.

©2019 Abushhaiwia, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.