MOJ
eISSN: 2374-6939


Case Report Volume 4 Issue 4
1Department of Medicine, Creighton University School of Medicine, USA
2Department of Medicine, University of Nebraska Medical Center, USA
3Department of Orthopedic Surgery and Rehabilitation, University of Nebraska Medical Center, USA
Correspondence: Carlos E Figueroa Castro, Division of Infectious Diseases, Department of Medicine, Creighton University School of Medicine, Omaha, NE 68105, USA, Tel (262) 836-7300
Received: February 24, 2016 | Published: March 18, 2016
Citation: Castro CEF, Smith PW, Daccarett MS (2016) Candida Glabrata Septic Arthritis Involving the Right Knee After Anterior Cruciate Ligament Graft Placement. MOJ Orthop Rheumatol 4(4): 00147. DOI: 10.15406/mojor.2016.04.00147
Anterior cruciate ligament (ACL) injury is the most common injuries involving the knee. Bone-patella tendon-bone (BPTB) allograft repair is an option for ACL reconstruction. Infectious complications after ACL reconstruction with allograft are rare. We present a case of Candida glabrata septic arthritis after ACL reconstruction with a BPTB allograft.
Keywords: Candida Glabrata, Osteomyelitis, Anterior Cruciate Ligament, Bone-patella tendon-bone graft
ACL, Anterior Cruciate Ligament; BPTB, Bone-Patellar Tendon-Bone; IVDU, Intravenous Drug Use; PCL, Posterior Cruciate Ligament; CDC, Centers for Disease Control and Prevention; NSAIDS, Non steroidal Anti-Inflammatory Drugs; FDA, Food and Drug Administration
Anterior cruciate ligament (ACL) injury is one of the most common ligament injuries involving the knee, especially in the young, athletic population. Bone-patellar tendon-bone (BPTB) and semitendinous gracilis autografts are some of the procedures used for ACL reconstruction. However, these procedures are associated with significant problems, including quadriceps weakness, patellofemoral pain, and patellar fracture, among others.1 Allograft ACL reconstruction is an attractive procedure, given the lack of donor-site problems, and more predictable graft sizes.1 However, it has its own disadvantages. Although uncommon, one of these disadvantages is the potential transmission of infections.2 Fungi are a rare cause of ACL allograft infection.3 Here, we present a case of Candida glabrata osteomyelitis after ACL allograft repair with a BPTB graft.
A 24-year-old white male sustained a right medial meniscal tear and anterior cruciate ligament (ACL) injury in January 2006. He was wrestling with some friends over a hard surface (concrete), when he sustained a twisting type injury to his right knee and felt a pop. He underwent a right arthroscopy for ACL reconstruction with a bone-tendon-bone cadaveric ACL allograft on September 16th, 2006. We could not determine information on antibiotic prophylaxis. His rehabilitation was unremarkable until January 2007, when he developed pain in his right knee, a new pop on his knee, with progressive swelling and joint effusion. He received a trial of physical therapy and nonsteroidal anti-inflammatory drugs (NSAIDs), with no resolution. He was evaluated by his orthopedic surgeon, who performed an arthrocentesis, relieving the swelling and pain. The fluid was not cultured. However, the swelling and the pain came back. Two more arthrocentesis were performed on February 5th and 15th. Both cultures from synovial fluid recovered Candida glabrata, which was believed to be responsible for his effusion and knee pain (cell count and differential were not available). He was started on IV caspofungin (loading dose: 70mg, maintenance: 50mg IV every day), with persistent swelling, pain with constant range of motion, and instability in his knee. He denied any numbness or tingling of the leg. He denied any other aches, bumps, and bruises. He also denied any current fever, chills or night sweats. His past medical history is unremarkable. He was not taking any medication in addition to caspofungin. He did not have any allergic reactions. He worked as a welder. He smokes two to three cigarettes per day, and denied alcohol or intravenous drug use (IVDU). His physical examination revealed a well-developed white male in no acute distress. His right lower extremity revealed a moderate effusion of the right knee, with considerable soft tissue swelling and mildly warmer than the left knee. Range of motion was from full extension to approximately 115 degrees flexion. The knee has a positive Lachman sign, the McMurray test was difficult to assess, and muscle strength was normal. He had a brisk capillary refill, and conserved sensation to light touch throughout his extremities. Surgical incisions from previous arthrocentesis did not show evidence of infection. Except for mild thrombocytosis (platelet count: 430x106/mL), complete blood count and basic chemistry were within normal limits. Erythrocyte sedimentation rate was 35mm, and C-reactive protein was 2.5mg/dL. An MRI showed a chronically ruptured ACL graft, with resorption of the graft, proximal retention screws extending into the joint space, and joint effusion (Figures 1-3). X-ray imaging showed osteolysis at the level of the tibial tunnel. An Infectious Diseases consultation was obtained, after concern for C.glabrata septic arthritis and possible osteomyelitis. Caspofungin was continued, and a new arthrocentesis was performed (cell count or differential were not performed).
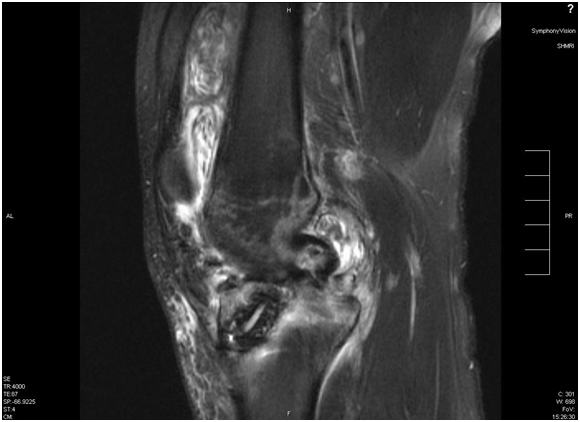
Figure 3 Large effusion with multiple loose bodies on suprapatellar pouch and septum formation. Tibial tunnel with surrounding edema in bone is seen.
After receiving cefazolin per perioperative antimicrobial protocol, he underwent right arthroscopy thru a standard anterolateral approach on March 12, 2007, with irrigation of the joint showing hundreds of white, discoid loose bodies of cartilaginous appearance loose bodies (Figure 4A-1C), from 0.9x0.6x0.2 cm to 1.0x0.8x0.2 cm. After copious irrigation with around 6L of 0.9% saline solution with epinephrine, the knee compartments were examined, with evidence of hypertrophic and bleeding synovium in the anterior medial and lateral compartments. The medial meniscus had a longitudinal tear, covered by hypertrophic synovium. The ACL graft tibial stump was covered by soft synovium, and it was completely friable and easy to break down with direct shaving. After removing the loose bodies using an arthroscopic shaver, a skin incision right over the tibial tunnel was performed. Bioabsorbable screws on the tibia were removed first and using ACL trephines and curettes, debridement of the tibial tunnel was performed. The femoral screw was removed from the femoral tunnel, and debridement of the femoral tunnel was performed, until healthy, normal cancellous bone was found. The remaining ACL graft was completely removed, and partial synovectomy of the compartments was performed. After removing all the visible parts, the knee was irrigated with 9L of 0.9% saline solution with bacitracin. The posterior cruciate ligament (PCL) was intact. His postoperative course was unremarkable. Joint fluid examination revealed an inflammatory joint effusion (white blood cell count: 17,265 per mL), with lymphocytic predominance (lymphocytes: 53%). The Gram stain from the joint fluid obtained prior to his knee surgery revealed many white blood cells, and the culture was positive only for few C.glabrata. This fungus was also recovered from the cultures from the loose bodies and the remaining ACL graft. An antifungal susceptibility testing was requested (University of Texas Health Sciences Center, San Antonio,TX). A new arthroscopic examination for irrigation and debridement was performed two days later, with direct shaving of the fibrous tissue of the anterior compartment, as well as new debridement of bone tunnels without finding any signs of infection or remaining loose bodies. The medial and lateral meniscuses were explored, and a partial lateral meniscectomy was performed. A very vascular synovium was found covering the lateral meniscus, so a partial meniscectomy on that layer, as well as the suprapatellar region, were performed. The knee was again irrigated with 9L of 0.9% of saline solution with bacitracin. While the patient was in the hospital, IV micafungin 100mg every day was used instead of caspofungin, due to formulary restriction. He restarted IV caspofungin 50mg every day after discharge. Two weeks later, the patient described decline in swelling, with absent pain, and able to bear weight on his knee. The antifungal susceptibility testing had the following MIC values (mcg/mL): Amphotericin B: 0.25, fluconazole: 4, itraconazole 0.5, voriconazole: 0.25, caspofungin: 0.25, micafungin: ≤0.03. He was switched to oral voriconazole 200mg twice a day. Based on the MIC value interpretation by the Clinical Laboratory Standards Institute,4 this isolate is susceptible to voriconazole. The patient was last seen four months after his knee surgery, with no symptoms.
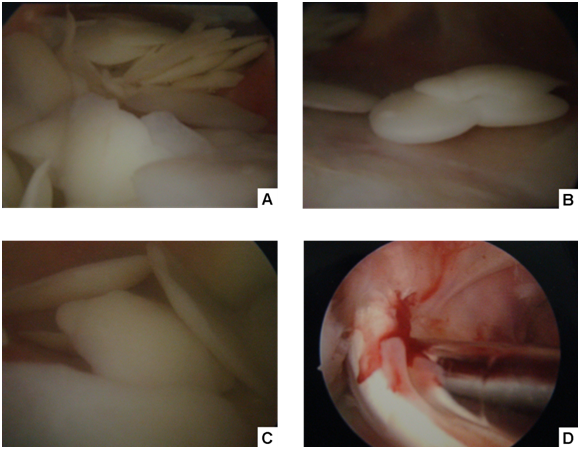
Figure 4 Arthroscopic view from anterolateral portal of the right knee, showing multiple small discoid loose bodies on suprapatellar pouch (snow storm appearance).
The Centers for Disease Control and Prevention (CDC), Division of Healthcare Quality Promotion, and the allograft tissue bank were contacted to investigate potential cases of fungal infections related to the donor ACL graft. An internal investigation by CDC did not reveal other cases of infection associated with the donor graft (Cynthia Lucero, personal communication).
Candida is a family of dimorphic fungi, which are common colonizers of the skin and mucous membranes. It is found in the environment on leaves, flowers, water and soil. The genus Candida includes approximately 154 species. Six of them are associated to human infections: C.albicans, C.tropicalis, C.glabrata, C.parapsilosis, C.krusei, and C.lusitaniae. C.albicans is most common yeast, but there is evidence of increase recovery of non-C.albicans isolates in human infections. Patients with immune compromise, indwelling central venous access, prior exposure to broad-spectrum antibiotics, and IV drug abuse, are at high risk of infection with Candida species. Usually, non-C.albicans isolates more resistant to azole therapy (e.g.: fluconazole). Progressive increase in invasive candidal infections is felt to be due to the increase in the number of high-risk patients such as hematologic stem-cell transplantation, the idespread use of central venous access for medication administration, the use of total parenteral nutrition which may provide a good growth medium for Candida, the excessive use of broad-spectrum antibiotics (which eliminates competing bacterial flora, and IV drug use.5 Azole-resistant isolates are increasingly common in an era of widespread azole use.
Candidal osteomyelitis and septic arthritis are rare events. The mechanism of infection is more often related to hematogenous seeding in patients with fungemia. Other sources of infection include exogenous inoculation after trauma, intraarticular injection, or prosthesis implantation. The clinical manifestations are subtle, and often occur months after the episode of fungemia or the surgical procedure. A high index of suspicion is required to establish the diagnosis, since the clinical, laboratory, and radiologic findings are nonspecific. According to the Infectious Diseases Society of America guideline on treatment of candidiasis,6 candidal osteomyelitis and arthritis are best treated with combined surgical debridement of the affected area and antifungal therapy. The recommended surgical treatment is the removal of all prosthetic material and necrotic bone. If placement of a new prosthetic material is required, a two-stage procedure is recommended. Because the diagnosis is often delayed for weeks to months, the infected bone is often poorly perfused, limiting delivery of antifungal medications. In addition to surgical debridement, multiple regimens include amphotericin B at 0.5 to 1mg/kg/day for 2 to 3 weeks, followed by fluconazole 400mg daily for 6 to 12 months; amphotericin B 0.5-1mg/kg per day for 6-10 weeks; amphotericin B with or without flucytosine; and fluconazole (for susceptible isolates) at 6mg/kg per day for 6 to 12 months. C.glabrata is a rare cause of osteomyelitis and septic arthritis, as reviewed elsewhere.5,7,8
Although amphotericin B has been the classic option for azole-resistant isolates, side effects are problematic, and echinocandins are a new parenteral option for treatment of fungal infections. The mechanism of action involves the inhibition of beta-glucan synthesis in the fungal cell wall. We could find only four references to the use of Echinocandins in Osteomyelitis.9-12 Information on echinocandin levels in bone is lacking, but might be an attractive medication because of the low likelihood of side effects when compared to Amphotericin B.
According to the American Association of Orthopedic Surgeons, around 95,000 ACL injuries are treated per year in the United States, with an estimated cost of $1 billion.13 Infection from a contaminated allograft if less common, but has been reported. The exact number of persons who develop septic arthritis caused by bacterially contaminated allografts is unknown. Reporting of infections resulting from contaminated allografts is not required. The estimated prevalence is 0.3% to 0.42%.3,14 The most common isolates are Staphylococcus aureus, Staphylococcus epidermidis, Peptostreptococcus sp., or a combination of these isolates, which suggests the patient’s own skin as the source of infection. In 2004, U.S. tissue banks distributed more than 1.5 million of allografts for transplantation.2 Tissue banks, donors, and recipients often are located in different states, complicating detection of bacterial infections associated with contaminated allografts. Food and Drug Administration (FDA) has proposed regulations that would require reporting adverse reactions that involve the transmission of communicable diseases if fatal, life threatening, or results in permanent impairment. The FDA requires screening of tissue donors for HIV, hepatitis B and C, and other bloodborne pathogens.
Indelli et al.15 reviewed 3500 ACL reconstructions. Six post-operative infections were identified, with an average onset of symptoms of 7.5 days. Staphylococcus aureus was isolated in two cases, Staphylococcus epidermidis in two cases, and non-hemolytic Streptococcus in one case. All cases received treatment for 6 weeks of IV antibiotic. Four of six grafts were retained. The rate between autografts and allografts was the same. In a CDC report, the Minnesota Department of Health received a report of three previously healthy patients who died following uncomplicated knee surgery; two had total knee arthroplasty, and one had cartilage graft implantation. All fell ill one to four days postoperatively. The symptoms include severe abdominal pain, and hypotension. The course of the disease was associated with septic or cardiogenic shock, with death within 24 hours of symptoms onset. After 5 days of incubation, a blood culture from one patient yielded Clostridium sordellii. The causes of death were unknown.16,17 Similar cases have been reported in Florida,18 Louisiana,18 and Colorado.19 In an investigation in California, Crawford et al described an outbreak of eleven patients that had infections following ACL reconstruction.13 Interestingly, two of the cases involved C.glabrata. We have found another case of fungal infection after ACL reconstruction,3 with the isolation of an organism described as Phycomycoses, from the order Mucorales. The treatment involved amphotericin B for six weeks.
In summary, we presented a case of C.glabrata septic arthritis after ACL reconstruction with a BPTB allograft. ACL allograft infections are an uncommon complication of allograft ACL repair, and fungal infections are an extremely rare cause of infection. There is no consensus in the management of this condition, but it involves prolonged antifungal infection, surgical debridement of bone tunnels, graft salvage or resection in certain situations, and open or arthroscopic synovectomy of the joint.19-21 High index of suspicion is crucial, especially if signs and symptoms of septic arthritis after ACL allograft placement without isolation of bacteria, or worsening symptoms despite appropriate antibacterial therapy. Candidal osteomyelitis is a difficult condition to treat, and Candida needs to be considered in the differential diagnosis of infection related to ACL allograft repair. The isolation of non-C.albicans isolates is on the rise, and the complete species identification plays a role in deciding the right antifungal treatment, in addition to surgical debridement. It is important to investigate the donor of the graft in collaboration with the tissue banks and the public health authorities, in order to avoid contamination of more patients.
None.
None.

©2016 Castro, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.