MOJ
eISSN: 2374-6939


Review Article Volume 5 Issue 3
Department of Orthopaedics, Athens University, Greece
Correspondence: Antony Vasileiadis, 13b Kyklaminon street, Gerakas, Athens Greece, Tel 2106080742
Received: June 20, 2016 | Published: August 9, 2016
Citation: Vasileiadis A (2016) Bridging of Peripheral Nerve Defects by Autologous Nerve Grafting Personal Experience. MOJ Orthop Rheumatol 5(3): 00183. DOI: 10.15406/mojor.2016.05.00183
The majority of peripheral nerve injuries occur in the upper limb and are from traumatic causes.1 These injuries disproportionately affect young healthy civilians and military officers who are most at risk of traumatic injuries.1 Severe nerve injury has a devastating impact on a patients’ quality of life. Typical symptoms are sensory and motor dysfunction that can result in complete paralysis of the affected limb or development of neuropathic pain.2 Nerve fibers of the transected nerve regenerate spontaneously to the extent limited by the size of the nerve gap, neuroma, and scar tissue formation.2
The primary goal of nerve repair is to allow reinnervation of the target organs by guiding regenerating sensory, motor, and autonomic axons into the environment of the distal nerve with minimal loss of fibers at the suture line.4
When a nerve has been injured, the aim of surgical repair is generally to re-approximate the ends of the injured nerve. Sometimes during repair after a nerve injury, a portion of the injured nerve, called a neuroma, needs to be excised, leaving a gap. When the nerve ends cannot be brought together, then a nerve graft may be necessary.
Definition of nerve grafting
Replacement of an area of defective nerve with a segment from a sound one (Figure 1).

Figure 1 Nerve grafting. (Reproduced with permission from the Mayo Foundation for Medical Education and Research).
Indications for nerve grafting
Primary nerve repair includes repairs carried out within 1 week of injury. Any repair carried out later is termed secondary.5 Nerve grafting is indicated to bridge a defect when greater than 10% elongation of the nerve would be necessary to bridge the gap.
4 cm is often used as the critical defect for grafting in the limb.
Role of the nerve graft
The autogenous nerve graft provides a source of empty endoneurial tubes through which the regenerating axons can be directed. It also provides a source of viable Schwann cells.
To be effective, the graft must acquire a blood supply. If the nerve graft survives, the Schwann cells also survive.6,7
Types of nerve grafts
Trunk graft: The trunk graft technique uses a segment of a nerve to approach the two stumps of the injured nerve. The main disadvantage of the technique is the poor revascularization of the core of the graft (Figure 2).
Cable graft: When several parts of a nerve graft are gathered together by sutures or glue. In this way, someone can then have a “nerve” of the same diameter to suture it with the target nerve stump (Figure 3).
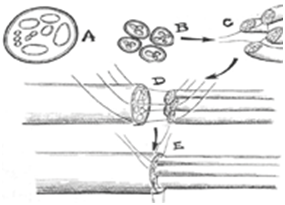
Figure 3 Cable graft.11
Pedicle graft: This technique of grafting is used when both median and ulnar nerves are damaged. The ulnar nerve is used as a pedicled (vascularized) graft to repair the median nerve lesion.
Interfascicular graft: A technique described by Millesi.8 It utilizes strands of grafted nerve between groups of fascicles (Figure 4).
Free Vascularized graft: The main application of this type of graft is in surgery of brachial plexus injuries, when there is an avulsion lesion of the C8 and T1 roots. In such cases the ulnar nerve can be used either as pedicled graft or as a free vascularized one (Figure 5).
Commonly used donor nerves
Upper extremity grafts
Lower extremity grafts
The use of a donor nerve results in a sensory loss in its distribution. This area of sensory loss becomes smaller over 1-3 years with collateral sprouting from the surrounding sensory nerves.
Graft Diameter
Small-diameter nerve grafts spontaneously revascularize, but large-diameter grafts do so incompletely. Thick grafts undergo central necrosis with subsequent endoneurial fibrosis (Figure 6).
Factors influencing the result
Time since the injury: Peripheral nerve injuries requiring surgical intervention will have better results the earlier the nerve is repaired after injury
Type and extent of injury: The more localized and confined the injury, the less trauma to the nerve, and the shorter the required nerve graft, then the better the outcome.
Vascularity in the area: For a nerve graft to be successful, it must be revascularized quickly. Therefore, adjacent healthy soft tissues will help the graft to be revascularized. The first few days after grafting, cellular viability is dependent solely on diffusion from the tissue bed.
Orientation of graft placement: It is important to place a nerve graft so that it is oriented in the same functional direction from which it was harvested. Axonoplasmic flow should be maintained in the same direction. A plan is made concerning which part of the proximal stump should be connected with the distal stump (aimed connection). Aimed connection is important in distal levels, where nerve fibers are arranged according to function.
In interfascicular grafting each end of a graft has to be approximated to one large fascicle or fascicle-group. This is done by using one 9-0 or 10-0 suture between the graft and the fascicle or group of fascicles.
The shorter the nerve graft required, the better the result. This is due in part to the amount of time it takes for regeneration to occur across each anastomosis area (7-14 days) and along the length of the nerve (0.2–3 mm per day).
Quality and type of repair: Quality of repair is directly depended on the surgeon's skill and experience.
Tension on repaired nerve: The nerve should be grafted with no tension on the nerve segments and areas of anastomosis. A harvested nerve graft shrinks in length by approximately 20%, and additional length may be lost in final preparation of host and nerve graft ends.
Preparation of the host nerve: A good result requires removal of the area of injury and assurance of healthy, viable nerve at the proximal and distal stumps. The neuroma developed at the proximal stump, must be resected. Resection of the nerve stump is continued until normal nerve tissue is reached (Figure 7). (From Midha R, Lee P, Mackay M. Surgical techniques for peripheral nerve repair. In: Wolfa CE, Resnick DK, eds.Neurosurgical Operative Atlas, 2nd ed. Spine and PeripheralNerves. NewYork: Thieme, 2007, pp 402-408)
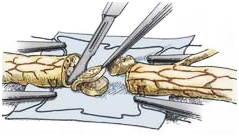
Figure 7 Preparation of the host nerve.12
Age of patient & other health factors: Children have a greater ability to centrally adapt to altered nerve programming, greater regenerative capabilities, and greater healing and metabolic rates than older patients.
Graft Incorporation: When separated from its blood supply, the graft undergoes Wallerian degeneration. Schwann cells can survive 7 days, depending purely on diffusion. The length of the graft is of no significance to the end result, provided that there is a tension-free anastomosis.9
Autograft disadvantages
Complications
The ideal nerve graft
Wallerian degeneration is the predictable sequence of events that occurs in both the axonal process and cell body after axon division. It occurs distalto the point of severance.7 This process clears the Schwann cell tubes in preparation for regenerating axons. Calcium-dependent proteolytic enzymes begin the process by breakdown of axonoplasm components into granular material.10 The ability of the distal axon to transmit action potentials diminishes as the process continues. Motor conduction is absent after approximately 9 days and sensory conduction after 11 days.10 Macrophages from the Schwann cell and the peripheral circulation phagocytize myelin. The macrophages contain peripheral markers, which are a powerful stimulus to NGF production by the Schwann cell. After myelin is cleared, the individual Schwann cells proliferate within the original myelinated tube, forming a continuous line of cells called Büngner's bands. The Schwann cells also have surface markers that are favorable to axon growth.
In a period of twelve years, 28 patients were treated for upper limb peripheral nerve lesions with various degree of nerve substance loss.
In all cases I used the same principles of nerve grafting, performing the following steps.
The two nerve stumps were identified and prepared. Any neuroma developed at the proximal stump was removed until normal appearance of normal nerve tissue (fascicular structure, bleeding, and no fibrosis of the endoneurium). I use an old fashion razor blade to remove the damaged segments of the stump.
If the nerve consists of more than one fascicles the perineurium must be dissected longitudinally and elevated so we can observe the fascicular pattern of the nerve. To facilitate the coaptation of the graft the fascicles are transected at slightly different level. Once the two stumps of the nerve are prepared, the length of the gap is measured.
At this point, I always release the tourniquet and perform thorough hemostasis. In most cases I used the sural nerve as a donor, because it is a reliable long nerve which can provide several segments of equal width. The graft must be longer than the measured length of the defect because it shrinks in length by approximately 20%, and additional length may be lost in final preparation of host and nerve graft ends.
The grafts are introduced and placed along the defect and approximated one by one with a 9-0 nylon suture. The graft ends are then manipulated in such a manner in order to achieve the optimal coaptation. A second suture is used only if the graft tends to rotate.
The wound was always closed carefully with the limb in the same position in which it was during grafting. The limb is immobilized in exactly the same position for 8 days. Peripheral nerve trauma treated by autologous nerve grafting: 2001 – 2012 (N = 28) (Table 1&2).
|
M.R.C. |
No |
% |
|
M4 |
6 |
37.5 |
|
M3 |
6 |
37.5 |
|
M2 |
4 |
25 |
|
S4-S3+ |
8 |
50 |
|
S3 |
5 |
31.2 |
|
S2 |
3 |
18.8 |
Table 1 Functional results of Median Nerve grafting in 16 patients
|
M.R.C. |
No |
% |
|
M4 |
6 |
50 |
|
M3 |
3 |
25 |
|
M2 |
3 |
25 |
|
S4-S3+ |
6 |
50 |
|
S3 |
4 |
33.3 |
|
S2 |
2 |
16.7 |
Table 2 Functional results of Ulnar Nerve grafting in 12 patients
Patient P.D.
Left forearm injury primarily operated in a district Hospital by direct suturing of the ulnar nerve. On admission in our Department, 4 months later there was complete absence of sensory and motor function of the ulnar nerve (Figure 8).
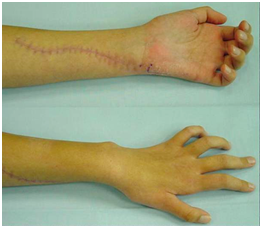
Figure 8 4 months later there was complete absence of sensory and motor function of the ulnar nerve.
The ulnar nerve was explored, a large neuroma at the site of suture was found, which was resected and the defect bridged with three nerve grafts (Figure 9).
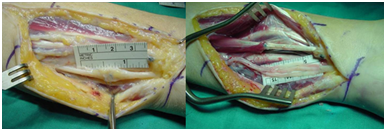
Figure 9 The ulnar nerve was explored, a large neuroma at the site of suture was found, which was resected and the defect bridged with three nerve grafts.
The postoperative result after one year was restoration of sensation and function of the interosseous muscles (Figure 10).
Nerve grafting is knowledge of the last one hundred years that is enlarged by experience and limited only by the imagination.
A great number of factors may influence the type of graft and manner in which it is used. A profound knowledge of the intra fascicular topography of peripheral nerves, in association with intra operative aids for motor and sensory differentiation, can lead to superior clinical results, especially with large nerve deficits.
The basic dogmas of managing a nerve gap will undoubtedly remain in our minds until the results of reinnervation through the use of synthetic conduits can reliably match the time-tested standards of the autogenous nerve graft.
None.
None.

©2016 Vasileiadis. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.