MOJ
eISSN: 2374-6939


Mini Review Volume 9 Issue 4
Department of Orthopaedics, General Hospital G Papanikolaou, Aristotle University of Thessaloniki, Greece
Correspondence: Konstantinos Ditsios MD PhD, Assistant Professor, First Orthopaedic Department, Aristotle University of Thessaloniki, G. Papanikolaou General Hospital, Exohi, GR 57010 Thessaloniki, Greece
Received: November 13, 2017 | Published: December 8, 2017
Citation: Kostretzis L, Pinto I, Konstantinou P, Karavelis A, Theodoroudis I, et al. (2017) Basic Science in Rotator Cuff Tears. MOJ Orthop Rheumatol 9(4): 00365. DOI: 10.15406/mojor.2017.09.00365
Rotator cuff (RC) tears are the most common soft tissue injuries of the shoulder. Despite the surgical improvement with new techniques in the repair site of the tears, the clinical results remain with high rate of failure. Deferent experimental studies in many animal models try to evaluate the complexity of the clinical problem. In this review article we summarize the knowledge from studies regarding the tendon to bone repair and healing, the early inflammatory reaction, the bone, tendon and muscle condition and the diversity of scaffolds that have been used in order to bridge a gap between RC tendon and the recipient site on bone. To answer the spectrum of questions regarding the treatment of RC tears further specific knowledge using the aforementioned animal models is compulsory.
Keywords:rotator cuff tear, tendon to bone healing, animal model, mini review, basic science
Rotator cuff (RC) tears are one of the most common clinical problems of upper extremity causing shoulder pain and functional loss. The impact of this condition to the socio-economic status of the society is quite significant and cannot be overlooked. In 2002 alone, approximately 40,000 surgical operations were performed in USA at the cost of $14,000 per surgery. There is an ever-growing increase in the number of surgical interventions of RC tears and RC repairs remain as one of the most common operations performed.1
More than 18million people report shoulder pain in USA.2 Overall RC tear prevalence is estimated up to 19% of the population, especially in patients over 60years old it can be as high as 50%.3−5 The therapy for full thickness RC tears includes conservative and surgical treatment, but despite new techniques and new fixations, the results remain suboptimal.6,7 Over the past few years a lot of basic science articles were published regarding etiology, therapy and postoperative treatment of RC tears.
It is well known that both intrinsic and extrinsic mechanisms have been accused of causing rotator cuff tendon injury and tears. Nonetheless, primary intrinsic degeneration of the tendon better interprets RC pathology.
There are several factors that predict the risk for symptoms: tear size, symptoms, location, age, activity level, muscle quality and nerve function is among the most important of them. Animal models are being used to enhance our understanding of the role of these factors to RC pathology and to find predictors for a successful RC repair.
Tendon condition
There is plethora of animal and cadaveric studies assessing the tendon bone fixation and healing. On different animals studies the authors show not only how the tendon behaves after a massive tear in the course of time but also how the tendon heals to bone in acute and chronic tendon RC tears. Furthermore, on cadaveric studies the strength of tendon to bone fixation and the anatomy of the area are evaluated.
There are different methods to repair full thickness RC tears with the most common being the double row (DR) technique. Recently Fei W et al.,8 showed, in their animal studies, better biomechanical and histological results with suture bridge repair (SB) as opposed to double row repair. The authors showed that the SB suture method provided better anatomical reduction of the RC footprint and better healing with more compact collagen fibers extending naturally from the tendon to cancellous bone. The difference in morphology of the bone-tendon interface may be attributed to the difference in bone-tendon contact pressure.
The RC repair site responds initially with inflammatory reaction and the first 3days are characterized by multinucleated cells, whereas lymphocytes and plasma cells present in days 7 to 10.9−11 Angiogenesis starts from day 3 to 10. The repair site becomes stiffer and the ultimate force increases during the postoperative period. Collagen I, collagen III, TGFbeta-1, mRNA, cell proliferation and density significantly increase at the 10th postoperative day and achieve a plateau by 56days.
On the other hand, the characteristics of the tendon to bone healing differ substantially from the normal tendon insertion site. The biomechanical and histological characteristics of the repair area remain inferior to the uninjured sites.9−11
Regarding the tendon to bone gap, a number of scaffolds have been used to bridge the tendon defect. Despite the complexity of healing tissue engineering, research is focused on different approaches as biphasic12 and triphasic scaffolds have13 been generated with multiple cell types, electrospun nanofibrous, polymer scaffolds with gradients in mineral content, to better mimic the tendon to bone insertion site. More recently Libner J et al.,14
presented a new mineralization protocol for creating a tendon to bone scaffold, attempting to provide more stiffness in the repair area using the mechanics of PLGA nanofiber scaffolds.14 Thomopoulos et al.,15 attempted to protect with primary immobilization the insertion site of RC repair to allow the expression of type III collagen, decorin and biglycan (extracellular matrix) until the insertion site reformed and then to start passive exercise. Peltz et al.,16−20 with their studies demonstrated that after 2weeks of passive motion, range of motion improved and joint stiffness decreased compared to immobilization The same authors published better results after short (2weeks) or moderate (4weeks) period of immobilization before starting the exercise program.
Bone condition
The bone condition indicates that bone loss may play a significant factor in poor healing. In order to resolve this problem there are few studies that investigate bone density and condition at the insertion site of RC. Galatz L et al.,21 examined the bone density at the greater tuberosity after acute and chronic RC tears with peripheral quantitative computed tomography. Their results indicated that bone density decreases faster in acute and slower in chronic RC tears. According to the authors bone loss may be the cause in poor tendon healing when a RC repair is delayed.
To improve bone density at the RC footprint and to stabilize its viscoelastic properties Cadet E et al.,22 worked on a rat model. The authors proved that bone density and stiffness in the footprint area were higher when the rats received bisphosphonates.
Muscle condition
Most of the experimental studies focus mainly in chronic RC tears because in these conditions there are mainly structural changes to the muscles. A variety of animals (mainly rats, rabbits, dogs and sheep) are used as experimental models to explore these changes.23−35 The dysfunction of chronic RC tears’ might not recover after an RC repair and Coleman’s36 designated this condition as ‘point of no return’ suggesting reconstructing the RC at early stages as acute tears. The different types of muscles fibers initially switch and later transform in fatty atrophy. An early repair of the RC tear led to a faster recovery of both muscle function and tendon elasticity compared to a more delayed repair.
To examine in vivo the muscle force and the histologic changes (Figures 1-3) in a 3-dimensional (3-D) manner, Ditsios et al.,36 used the established a rat shoulder model37 to evaluate massive rotator cuff tears by sectioning supraspinatus (SS) and infraspinatus (IS) tendons. The author showed that a low functional outcome in a repair of a chronic RC tear may be attributed to the decrease in muscle power during their repair and secondly to the higher degree of degeneration in their dorsal part.
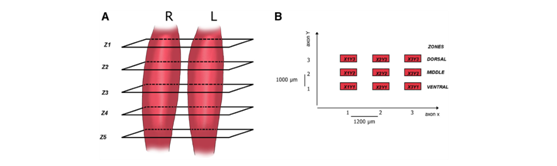
Figure 1A Transverse muscle sections were obtained at levels Z, localized every 6000 mm (Zk þ 1 1⁄4 Zk þ 6000 mm with k 1⁄4 1:5), where the first Z1 and the last level Z5 were at a plane 1000 mm from the lateral and medial ends of the detached muscle. B Nine optical fields (3 zones: ventral, central, dorsal) were taken at displacement steps of 1200 mm in the x-axis and 1000 mm in the y-axis. Each co- ordinate XiYj (i 1⁄4 1-3, j 1⁄4 1-3) corresponds to an optical field (873 628) mm.
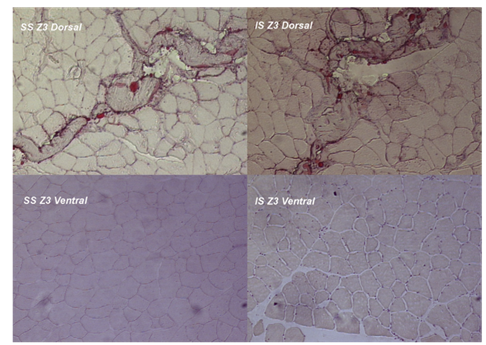
Figure 2 Oil red–stained photomicrographs from the midsubstance portion (Z3) of the surgical side supraspinatus (SS) and infraspinatus (IS) muscles. Photomicrographs are made at 10 lens of Carl-Zeiss light microscope (100 magnification). Greater fatty infiltration and atrophy of the dorsal part of both muscles compared with the ventral part at the same Z3 section.
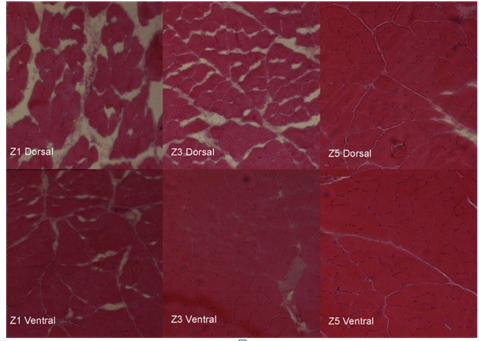
Figure 3 Hematoxylin-eosin–stained photomicrographs from the dorsal and ventral part of Z1 (lateral side, near tendon), Z3 (mid- substance), and Z5 sections of the supraspinatus muscles that were operated on. Photomicrographs are made at 10 lens of Carl-Zeiss light microscope (100 magnification). Degeneration was more prominent in the dorsal side of the surgical SS muscles and mainly at the sections near the tendon insertion (Z1, Z3), revealing a 3-D aspect of muscle atrophy.
Itoigawa et al.,38 studied the molecular mechanism of fatty muscle infiltration after an RC tear by investigating the expression of genes in vitro and then testing their expression profiles in vivo in a model. The author underlined the cardinal role of expression of gene Wnt10b which is suppressed when no mechanical muscle tension exists.
Further analysis of fatty infiltration in molecular level provided results of increased expression of adipogenic genes PPARc and C/EBPa, which when in absence adipogenesis is prohibited.39 Likewise the RC muscle atrophy is proportionally analogous to the size of RC tears.40−42
From the pharmacological view, anabolic steroids (nandrolone) group and insulin-like growth factor (IGF) were found to decrease the catabolic face of RC tears.43
Nerve - muscle function
Biomechanical and histological studies evaluate the structural components of RC muscles but these results do not explain how the nerves react with supraspinatus and infraspinatus muscles especially in chronic cases.44 A great number of studies demonstrates the nerve behavior and the complex couple excitation-contraction following RC tenotomy.45,46
Muscle dysfunction with chronic RC tears demonstrated with electromyographic studies show how the compound motor action potential (CMAP) amplitude46 decreases compared to the contralateral control shoulder. However EMG studies provide information only for the activated part of the muscle near to the electrode.47
In a recent study the authors developed a new technique for measuring the nerve reaction 4months after the initial cutting of supraspinatus (SS) and infraspinatus (IS) tendons in insertions site (Figure 4). Their data demonstrate that the muscles extensively lost mass especially in the tendon – muscle dorsal part area and the force decreased more 30% in SS and 35% in IS respectively.36
Consequently, there are many experimental studies describing the structural, histological, functional changing during time. Most of them emphasize in chronic massive rotator cuff tears and evaluate with CT, MRI, biomechanical, biochemical, electrophysiological, general and molecular histological and gene test but even with all that new knowledge there are many questions to answer in the futures.
Despite the plentiful of studies, there are many questions to address or to be elucidated regarding RC tears’ behavior during healing process. Certainly, animal models closely resemble the respective alterations observed in humans, and useful conclusions can be derived.
The authors confirm that this article content has no conflict of interest.
None.
None.

©2017 Kostretzis, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.