MOJ
eISSN: 2374-6939


Research Article Volume 2 Issue 1
1First Department of Orthopaedics, General University Hospital Attikon, Greece
2Hellenic Osteoporosis Foundation Kifissia, Greece
3Metabolic Bone Diseases & Rheumatology Unit, General University Hospital Attikon, Greece
4Laboratory for Research of the Musculoskeletal System, University of Athens Kifissia, Greece
Correspondence: Yannis Dionyssiotis, Fellow European Board PRM, First Department of Orthopaedics, General University Hospital Attikon, Athens, Greece, Tel 00306946469759
Received: November 07, 2014 | Published: February 19, 2015
Citation: Dionyssiotis, Lyritis GP, Skarantavos G, et al. Assessment of obesity with anthropometric and densitometry measurements in spinal cord injury. MOJ Orthop Rheumatol. 2015;2(1):42-46. DOI: 10.15406/mojor.2015.02.00037
Aim: Assessment of obesity by anthropometric and densito-metric measurements in spinal cord injury.
Materials and methods: Thirty-one subjects with complete spinal cord injury (AIS A) separated according to the neurological level in group A (n=16, high paraplegia: above the seventh thoracic neurological levels) and group B (n=15, low paraplegia) were compared with 33 controls. For the assessment of obesity we used body mass index (BMI) and dual energy X-ray absorptiometry (DXA, Norland) to study all subjects. Using the DXA method we calculated the total body fat in grams (fat mass).
Results: BMI values for paraplegic population were statistically lower compared to control group (23.9±3 and 26.2±4, respectively, p=0.025) and within the normal range of BMI. However, the comparison according to neurological level of injury revealed a significant difference between high paraplegics and controls (22.9±2.2 and 26.1±4, respectively, p=0.021). Using DXA fat was increased in body composition in paraplegics compared with controls (23071.38±9485 and 19015±6553, respectively, p <0.05). The correlation of BMI with fat mass was statistically significant paraplegics and controls (r=0.57, p=0001 and r=0.73, p=0.0001, respectively). In paraplegics total fat measured by DXA was increased at any given BMI value compared to the control group (r2=0.3 vs. r2=0.54, respectively). Further analysis between the two paraplegic groups showed a significant correlation between BMI and fat mass only in the group of low paraplegia (r=0.72, p=0.004).
Conclusion: The BMI is often used as a measure of obesity but assess body composition inadequately. The whole body DXA gives valuable clinical information regardless of the neurological level of injury.
Keywords: BMI, DXA, Spinal cord injury, Obesity, Fat mass
Obesity measurements become important as evidence identifies body fat as a significant predictor of mortality especially for SCI where carbohydrate intolerance, insulin resistance, lipid abnormalities, and heart disease, occur prematurely and at a higher prevalence in this population.1–4 Body mass index (BMI, kg/m2) was used in many studies as a surrogate measure of obesity. It is a very simple measurement of fat requiring only the measurement of height and weight; however it does not distinguish the individual components of weight and for this reason the applicability of conventional BMI cut off values is into question.5–7 In the meantime more sophisticated body composition technologies, i.e. dual-energy X-ray absorptiometry (DXA), for a more precise quantification of fat were introduced.Recently, DXA has gained acceptance as a reference method for body composition analysis.8,9 DXA software determines also composition, in this case fat mass, in different regions of the body being a three-compartment model.10 However, in clinical practice whole body DXA is not always available. The purpose of the study is to investigate whether is valuable to assess obesity by BMI versus dual X-ray absorptiometry in subjects with spinal cord injury.
Demographics
Sixty four Greek men were included in this study. Thirty one had a complete paraplegia (AIS A), according to the ASIA impairment scale.11 All were neurologically stabilized and at least 1.5 years post-injury. Total paraplegic population included subjects of Thoracic (T) 4-T12 neurological level of injury (mean age 39±16 yrs, height 1.76±0.07 m, weight 74.2±13 Kg) with a mean duration of paralysis 5.7±6 yearsin comparison with 33 healthy men as control group of similar age (37±19 yrs), height (1.76±0.05 m), and weight (81.36±13 Kg). (Table 1) Paraplegic men were also separated according to the neurological level of injury (NLoI) in group A which included 16 men with high paraplegia: T4-T7 NLoI, (mean age: 33±16 yrs, height 1.77±0.06 m, weight 72±8 Kg, duration of paralysis: 6±6 yrs), and group B which included 15 men with low paraplegia: T8-T12 NLoI, (mean age: 39±14 yrs, height 1.75±0.1 m, weight 76.7±13 Kg, duration of paralysis: 5.6±6 yrs). Paraplegics were volunteers recruited from the 2nd Rehabilitation department of National Rehabilitation Center “EIAA” in Athens (outpatients) and from the Greek Paraplegic Society after announcement for participation in a clinical research effort of Athens University. The control group consisted of volunteers working in the laboratory and the hospital. Anthropometric factors, including age, height, weight, BMI (in both paraplegic groups and controls) and clinical parameters such as age at injury, duration of paralysis were recorded in all paraplegics. Controls considered healthy after physical examination and medical history review. In Table 1 we present the anthropometric data and the clinical parameters of the study population and in Figure 1 inclusion and exclusion criteria. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research. This study was carried out in the 2nd Rehabilitation and Radiology departments of the National Rehabilitation Center "EIAA" in Athens, in cooperation with the Laboratory for Research of the Musculoskeletal system of Athens University (KAT Hospital) in Kifissia, Greece.
|
Subjects parameters |
Controls n=33 |
High Paraplegics |
Low Paraplegics n=15 |
ANOVA
|
|
Age (years ) |
37±19 |
35±14 |
39±14 |
0.370 |
|
Weight (kg ) |
81.36±13 |
76.67±17.12 |
76.67±17.12 |
0.085 |
|
Height (m ) |
1.76±0.05 |
1.77±0.06 |
1.75±0.10 |
0.676 |
|
BMI (kg/m2) |
26.12±5 |
22.94±2.21 |
24.86±3.50 |
0.02 |
|
Age at injury (yrs) |
● |
26.63±14.35 |
33.57±12.3 |
0.118 |
|
Duration of paralysis (yrs) |
● |
5.97±5.9 |
5.65±5.8 |
0.87 |
Table 1 Demographic data of the controls, high, low paraplegics and important paraplegics clinical parameters
Anthropometric measurements
In all spinal cord paraplegic subjects, the height was measured while in supine position before the examination. The controls’ height was measured with a wall mounted ruler in the standing position. Weight was measured on a standard weight scale in controls. In paraplegics, the subject’s weight was measured in seating position in the wheelchair after subtracting the wheelchair’s weight. BMI was calculated for each subject (BMI=weight (kg) /height2 (m)).
Dual-energy X-ray absorptiometry (DXA) measurements
All subjects were examined with a dual energy X-ray absorptiometry scan (DXA, Norland XR 36, Norland Corporation, Fort Atkinson, WI) for the estimation of FM (g) (Figure 2). The basic principles of DEXA are described elsewhere.10,12
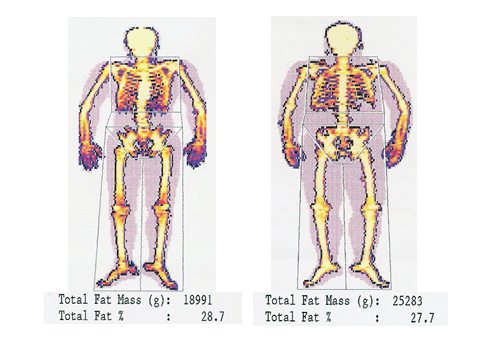
Figure 2 Whole body fat mass from paraplegic subject thoracic 6 (left picture) using whole body DXA (Norland X-36, Fort Atkinson, Wisconsin, USA) and values of measured parameters. Modified and translated with permission, courtesy of Dionyssiotis Y.
Statistical analysis
All variables are represented by the number of patients (n), mean value (mean), and standard deviation (sd). Comparisons of variables among the 3 groups were performed using the one way ANOVA and Bonferroni test for pair wise comparisons. Comparison of variables among the 2 paraplegic groups was performed using analysis of covariance model (ANCOVA) controlling for age at injury and duration of paralysis respectively. All tests are two-sided; p<0.05 was defined as significant. All data analysis was performed using the Statistical Package for Social Sciences (version 10.0) software (SPSS Inc., Chicago, IL).
BMI values were statistically reduced in paraplegics compared with controls (23.9±3 vs. 26.2±4, p=0.025, respectively). The comparison according to neurological level of injury revealed a significant difference between high paraplegics and controls (22.9±2 vs. 26.2±4, respectively, p=0.021). On the other site, using whole body DXA, values of fat mass in total paraplegic groups’ body composition compared with controls were increased (23071.4±9485 and 19015±6553, respectively, p<0.05). The correlation of BMI with FM was statistically significant in total paraplegic group and controls (r=0.57, p=0.001 vs. r=0.73, p=0.0001, respectively). After analysis of covariance, it was shown that paraplegics had more FM at any given BMI value than the able bodied subjects (r2=0.3 vs. r2=0.54 respectively) (Figure 3).
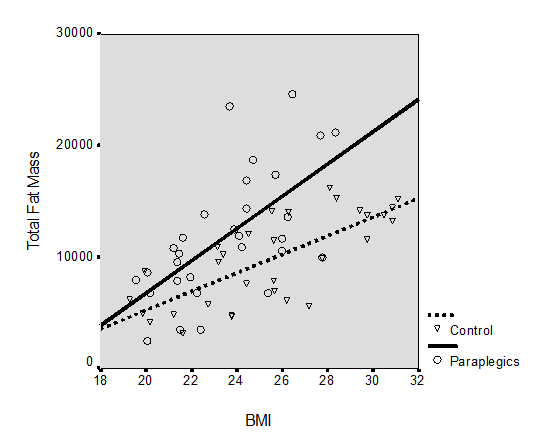
Figure 3 Relationship of total fat mass with body mass index (BMI) for controls and the two paraplegic groups.
Although, within the two paraplegic groups only the group of low paraplegics showed a significantly correlation of BMI and FM (high paraplegics: r=0.31, p=0.266 and low paraplegics: r=0.72, p=0.004) (Figure 4). Duration of paralysis (DoP) was correlated with total body fat mass only in high paraplegic group (r=0.476, p=0.073) (Table 2).

Figure 4 Relationship of total fat mass with body mass index (BMI) for the paraplegics and controls.
|
Correlations |
|||
|
Duration of paralysis |
High paraplegics |
Low paraplegics |
|
|
Total body – |
Spearman’s r |
0.476 |
0.136 |
|
p-value |
0.073 |
0.644 |
|
Table 2 Correlations between duration of paralysis and measured parameters in the two paraplegic groups
Body mass index (BMI, kg/m2) values in paraplegics and controls were found below values which signify obesity and within the normal range of BMI values in our study.13,14 This finding could be acceptable for the population of the controls who were examined, but raises questions regarding the paraplegics. Our controls were relatively young and we can’t exclude the possibility some of them to be really fit and sportive. According to the World Health Organization (WHO) a BMI of 30 kg/m2 is identified as the cut-off above which able-bodied people are considered obese.5 However, it is open to question if the cut-off points for underweight, normal, overweight, and obese patients used in able-bodied populations can be applied to SCI subjects. On the other side, there are recent studies reporting that up to 45% and 29% of SCI subjects are overweight and obese, respectively.14,15 In our study decreased BMI values in total paraplegic group were a puzzling result. However, mean BMI in studies of SCI subjects ranges from 23.1 to 25.7 kg/m2, which is in line with our results.4,5 Nevertheless, there are studies which demonstrate the usefulness of BMI as an indicator of obesity, in body composition in people with SCI.16 Whether the criteria of BMI may assess obesity in people with spinal cord injury the latest studies show the opposite.7 An explanation of the lower values of BMI in our population could also be the incidence of malnutrition-undernourishment in this population.17 Hyper-metabolism, catabolism and accelerated nitrogen loss are well-recognised complications that occur after traumatic spinal cord injury but this was not the case in our study because all paraplegics were in chronic stage after SCI. Similar body mass indices were found in BMI between paraplegics in the acute phase of injury and controls.5 In addition between paraplegics with high and low neurological level injuries in our study not statistically significant differences in BMI were highlighted. In other studies which included mixed populations BMI was found significantly higher in paraplegics compared to tetraplegics. Distribution of BMI by level of injury was similar with 37.5% and 40.5% of the male tetraplegic and paraplegic groups, respectively, falling into the recommended BMI range, 50% in each male group were overweight, and 12.5% and 10.8%, respectively, were classified as obese. Finally, fewer were obese compared with the able-bodied population.18 We need more evidence according to the impact of type and duration of the injury on the extent of obesity, cut-off points of obesity in SCI subject’s definitions and moreover adjustments in classifications of normal, overweight, obese, and morbid obesity by BMI for SCI subjects.18 All these findings highlighting the problem that using BMI fat is underestimated and it is an insensitive marker of obesity in subjects with SCI when measurements are compared with healthy subjects.3 In conclusion we believe according to our results that the reduced BMI in both groups with paraplegia reflects a result of a reduced lean mass (LM) in both paraplegic groups.
In our study only the group of low paraplegics showed a significantly correlation of BMI and FM. Low paraplegics had more FM at any given BMI value than the able bodied subjects. Spungen et al.4 found also a relationship of total body percent fat with BMI for a SCI and control group, but the finding that the correlation depends on low paraplegics’ values is new. The explanation lies in the specific alterations in paraplegics’ body composition and the influence of factors such as immobilization, damage of the sympathetic nervous system (SNS) nucleous and hormonal status which will be discussed in detail later in this paper.
Similarly to the healthy population values of BMI are positively correlated with obesity. This emerged from a study, conducted by whole body DXA Norland X-36, only when the findings of total fat in paraplegics were correlated with BMI. Using DXA was found that the total fat mass was statistically significantly higher for any given BMI value in paraplegics compared with controls,2 finding that strongly supports the studies held by the whole body DXA Hologic QDR-2000 method.4 All these studies illustrated statistically significantly higher total fat mass and fat percentages for any given unit of body mass index in paraplegics in comparison to controls. Increased fat per body mass index unit was found in a study of monozygotic twins, one with SCI compared with a non-SCI co-twin by the above authors also.19 However, when data from the analysis undertaken in areas measured by the method of whole body DXA were compared in the same patients there were differences between paraplegics with high and low neurological level of injury. This finding is new and reinforces those views on the inability of BMI usage in the analysis of body composition of paraplegics.2
As far as the fat mass is concerned, analysis of body composition with DXA has revealed large increases in fat in people who do not appear to be obese, yet they carry large amounts of fat tissue and in the group of paraplegic subjects fat mass was 47% higher.5 The percentage of fat mass in subjects with low paraplegia was comparable to controls by 20%, although in higher neurological level of injury the rate of fat ranged from 30 to 36% in 37 patients with spinal cord injury studied using radioisotope methodology.20 In 133 men with chronic SCI higher values of fat mass in paraplegics’ upper limbs were found compared with controls.4 The increased rate of fat by 29% which was found in paraplegics with low neurological level of injury is substantially similar with respect to a former study (increased rate of fat in paraplegics 20%) that resulted from an overall group of paraplegics that also included high paraplegics.2,20 The difference is due to participation of high paraplegics in the analysis of their study which reduced the percentage. In another study which included mixed paraplegic population (complete and incomplete paraplegics) was found higher amount of absolute fat mass in paraplegics and was suggested that this was responsible for the significantly lower than controls percentage of LM (soft tissue not absolute muscle mass) in paraplegics’ arms, which is quite unusual if we think about the arm overuse conducted through wheelchair activities.4 On the contrary other analysis of LM and FM in paraplegics’ upper limbs and controls didn’t show any significant differences.2 However, when paraplegics were analysed according to the neurological level of injury, values of fat in high paraplegics’ upper limbs were similar to controls and lower, in comparison with values of fat in the low paraplegics group (not statistically significant). Also, the corresponding relationship concerning the fat revealed decrease in fat by 26.6% in the upper limbs of the high paraplegics compared with low paraplegics’ upper limbs. Taking in mind that the upper limb is an overloaded bone in paraplegics an explanation of this finding could be that high paraplegics are trying to overcome their handicap through an arm overuse (high mechanical forces) reducing regionally adiposity and inducing LM. So the question is if the higher FM values in low paraplegics’ arms are responsible for the higher values found in bone mineral density in this subpopulation.2 Adds fat bone? To answer this we refer the reader to the pioneer publication of Reid I.R. who states that a number of mechanisms for the fat-bone relationship exist and include the effect of fat mass on skeletal loading and an hormonal cataract, involving a web of interrelated regulatory pathways.21
In the group of high paraplegics a strong relationship between duration of paralysis and total body fat was found. Despite the similar paralytic effect on body composition and the similar duration of paralysis in both paraplegic groups of this study the explanation of that strong correlation may be due to the following reasons: Unlike high paraplegics subjects with lower paralysis levels perform at a higher frequency weight bearing activities like standing i.e. in standing frames, therapeutic walking (which is possible using gait orthoses) that results in increased energy consumption and obesity reduction. Nevertheless, there is also sympathetic nervous system (SNS) dysfunction after SCI in high level neurological injuries (mainly above T6) attributable to loss of supraspinal control that occurs with disruption of spinal cord pathways. It has been observed that the higher the level of the SCI, the greater the degree of clinical manifestations of SNS dysfunction.22
The hormone leptin is secreted by fat cells and help regulate body weight and energy consumption.23 Locally leptin preserves bone in a concept: the higher the fat mass is the stronger bones we need to support the greater soft tissue mass.21 According to this the amount of leptin in the circulation is positively correlated with the percentage of fat in people.24 In paraplegics, when compared with healthy subjects, higher levels of leptin have been found, possibly due to greater fat tissue storage.25 Multiple regression analysis showed that serum leptin levels in men with SCI correlated not only with BMI but also with the neurologic deficit. This finding supports the notion that decentralization of sympathetic nervous activity relieves its inhibitory tone on leptin secretion, because subjects with tetraplegia have a more severe deficit of sympathetic nervous activity.26 On the other hand centrally leptin causes bone loss. A blockage of the SNS (like in high level spinal cord injuries) may modify the secretion and action of leptin leading to bone anabolism. However, leptin’s peripheral effects predominate and possible increase the risk of obesity in paraplegic patients with high-level injury.21,27,28
Our paraplegic population was limited and there is a wide individual variability in body composition but the influence of this effect in the sample of this study was beyond the scope of this paper. It is also possible that low paraplegics to act in their lifestyle like high paraplegics, i.e. they are mostly wheelchair subjects. A critical question that arises is how to best proceed programmatically to promote optimal body weight and composition to reduce disease risk. Studies according to interventions for reducing the increase in adiposity in paraplegics are welcome.
We would like to thank Olga Lazoura and Eleni Kourkouveli for performing the whole body DXA measurements and all paraplegics and controls who took part in this study.
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.

©2015 Dionyssiotis,, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.