MOJ
eISSN: 2374-6939


Research Article Volume 3 Issue 4
Department of Orthopaedics, Zhongshan Hospital of Dalian University, China
Correspondence: Xiaobing yu, Department of Orthopaedics, Zhongshan Hospital of Dalian University, No. 6 Jiefang Street, Dalian, Liaoning province China, Tel 86-0411-62893145
Received: October 08, 2015 | Published: October 27, 2015
Citation: Xiaobing YU (2015) A Animal Study of Immunohistochemical Analysis of Sympathetic Nerve Fibers in Osteonecrosis of Femoral Head in Comparison with Osteoarthritis. MOJ Orthop Rheumatol 3(5): 00100. DOI: 10.15406/mojor.2015.03.00100
Objectives: Osteonecrosis of the femoral head (ONFH) was mainly due to alterations of bone vascularity. And noradrenaline (NA), as the neurotransmitter of the sympathetic nervous system (SNS), leads to the vasoconstriction by activating its α-Receptor. This study was to explore the nerve fiber density of the femoral head in the rabbit model of ONFH, compared with the rabbit model of osteoarthritis (OA).
Methods: Twenty New Zealand White rabbits were randomly divided into two experimental groups, ONFH group (n=10) and OA group (n=10). The rabbit model of ONFH was built by the intramuscular injection of methylprednisolone acetate, while the rabbit model of OA were built by performing unilateral joint surgery. The nerve fiber density and distribution in the femoral head was determined using an Olympus BH2 microscope.
Results: No significant difference was found in the number of sympathetic nerve fibers per square millimeter between ONFH and OA periosteal samples. And so did synovial samples. The number of sympathetic nerve fibers in the femur bone samples of ONFH rabbits was significantly less than that of OA rabbits (p = 0.036). The significantly fewer sympathetic nerve fibers were observed in the ligamentum capitis femoris samples from ONFH rabbits than from OA rabbits (p = 0.028).
Conclusion: ONFH might be preceded by an inflammatory reaction, and an inflammatory response might lead to arthritic changes in tissue samples, which in turn reduces the number of sympathetic nerve fibers.
Keywords: Osteonecrosis of the femoral head, Osteoarthritis, Sympathetic nerve fibers.
ONFH, Osteonecrosis of The Femoral Head; NA, Noradrenaline; OA, Osteoarthritis; SNS, Sympathetic Nervous System; PBS, Phosphate Buffered Saline; BSA, Bovine Serum Albumin
Osteonecrosis of the femoral head (ONFH) is a progressive nonbacterial localized bone disease in adulthood, and accompanied with a complete destruction of the femoral head and a severe secondary osteoarthritis.1-3 The disease is often asymptomatic at first, so it is difficult to diagnose ONFH early. The first symptom is usually a deep groin pain; it seems likely that the number of sympathetic nerve fibers is increased.4 usually as a result of an accompanying bone marrow edema. Here, the motion of the hip can be still definitely inconspicuous in the clinical investigation.5 The first movement restrictions are evident in the internal rotation and abduction of the hip joint.6
ONFH are usually affects people aged between 30 and 50 years.7-11 150000-200000 new cases of ONFH are reported in China alone each year, so that the disease has a major role in the cost of health care.12 The etiology of the disease is still unclear. We only know several risk factors, including the prolonged use of corticosteroids, alcohol abuse, smoking, sickle cell anemia and femoral neck fracture.13,14 Hemodynamic response is key point. It is considered that the cause of ONFH is based on an altered blood flow situation of the bone.15,16 Atsumi et al.17 examined the vascular situation in ONFH patients by angiography. They found that there is no radiological necrosist onset, missing the vessels in the capsule of the upper femoral neck completely.17 But these are just responsible for the vascularization of the greater part of the femoral head and the nerve fiber density of the femoral head as already Sevitt et al.18 has described.
To understand the etiology and pathogenesis of ONFH better, this study was to explore the nerve fiber density of the femoral head in the rabbit model of ONFH, compared with the rabbit model of osteoarthritis (OA). Therefore, it seems necessary to explore early diagnosis and more promising treatment methods.
Animal
Twenty adult New Zealand white rabbits were used in the study. Animals were housed in separate cages in an air-conditioned room. The rabbits were free access to drink water, and fed a commercial rabbit diet. All rabbits were randomly divided into two experimental groups, ONFH group (n=10) and OA group (n=10). All animals received humane care in compliance with the Principles of Laboratory Animal Care formulated by the National Society of Medical Research. The protocol was approved by the Animal Care and Use Committee of Southern Medical University.
ONFH and OA model
The steroid-induced ONFH rabbit model was built as described previously.15 A 20 mg/kg body weight dose of methylprednisolone acetate (MPSL; Upjohn, Tokyo, Japan) was administered once into the right gluteus medius muscle of all the rabbits in ONFH group to induce ONFH. Four weeks after the administration of MPSL, the rabbits were sacrificed and tissue samples were prepared. The rabbit model of OA were built, grooves were surgically made in the articular cartilage of left hip. The femoral condyles of both knee joints in OA group as described previously,16 while the right joint was left intact.
Sample preparation
After the rabbits were sacrificed, the samples of femur bone, periosteum, femoral head, synovium and ligamentum capitis femoris were collected from both ONFH and OA group. The samples were placed in phosphate buffered saline (PBS) supplemented with 4% formaldehyde (Merck, Denmark) for 12-24 fixed hours. Then, samples were incubated in PBS containing 20% sucrose for 12-24 hours. The next day, the samples were embedded in OCT compound (Tissue Tek; Sakura Finetec), and snap-frozen in liquid nitrogen at -80 °C.
Immunohistochemical evaluation
The frozen samples were sectioned at 8 μm in a cryostat microtome, and the frozen sections were put onto the Super frost Plus slides (Menzel-glasses, Braunschweig, Germany). Subsequently, samples were incubated for 50 min in a blocking solution consisting of 10% bovine serum albumin (BSA), 10% chicken serum, and 10% FCS (Sigma, Deisenhofen, Germany) and then pipetted on. Such a block solution was used to prevent nonspecific binding of antibodies directed against tyrosine hydroxylase in sympathetic nerve fibers. Thus, a nonspecific background staining can be avoided. The samples were washed with PBS, and then were incubated at 4 °C overnight in rabbit polyclonal anti-TH (Chemicon, AB152) at a dilution 1:250 in PBS with containing 0.3% Triton and 10% goat serum. By the antibody binding to the enzyme, and thus it marked the sympathetic nerve fibers. The nerve fiber density was determined using an Olympus BH2 microscope. From each sample, 17 fields of view were counted. The nerve fibers were counted and the mean of these structures is converted to the area of 1 mm2. A nerve fiber was considered positive when a beaded structure was represented with at least three members, or a length of 50 microns could be measured using a small scale within each visual field.
Statistical analysis
The data were represented by box blots. The calculations were done using Sigma Plot (version 9.0). Comparisons between groups of data were performed by using a Student’s t-test or Mann-Whitney test. A p-value < 0.05 was considered to indicate statistical significance. Data were analyzed with the SPSS 18.0 statistical software package (SPSS Inc., Chicago, IL).
Figure 1A and Figure 2A showed the sympathetic nerve fibers in the periosteal and synovial sample of an OA rabbit, respectively. No significant difference was found in the number of sympathetic nerve fibers per square millimeter between ONFH and OA periosteal samples, shown in Figure 1B & Figure 2B. The number of nerve fibers was approximate in both ONFH and OA periosteal samples. The number of sympathetic nerve fibers per square millimeter was also countered in the synovium samples, and no significant difference was found between ONFH and OA synovial samples.
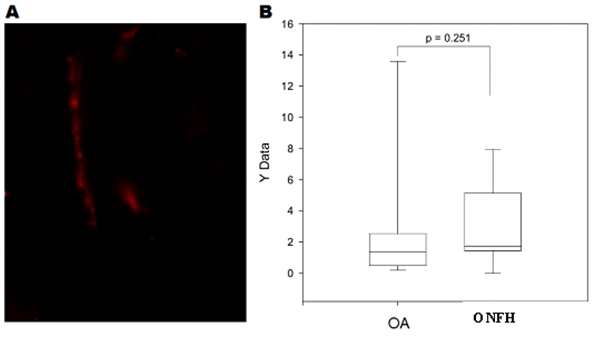
Figure 1 Sympathetic nerve fibers in the periosteal.
Figure 1A Sympathetic nerve fibers in the periosteal sample of a OA rabbit.
Figure 1B Comparison of the number of sympathetic nerve fibers per square millimeter between ONFH and OA periosteal samples (p = 0.251).
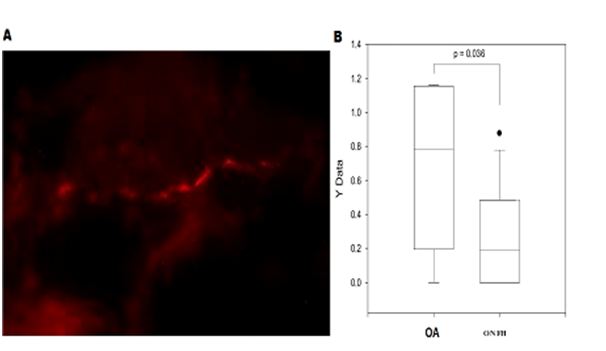
Figure 2 Sympathetic nerve fibers in the synovial sample.
Figure 2A Sympathetic nerve fibers in the synovial sample of an OA rabbit.
Figure 2B Comparison of the number of sympathetic nerve fibers per square millimeter between ONFH and OA synovial samples (p = 0.683).
Figure 3A showed the sympathetic nerve fibers in the femur bone sample of an ONFH rabbit. A significant difference was found in the number of sympathetic nerve fibers per square millimeter between ONFH and OA femur bone samples (p = 0.036), shown in Figure 3B. The number of sympathetic nerve fibers in the femur bone samples of ONFH rabbits was significantly less than that of OA rabbits.
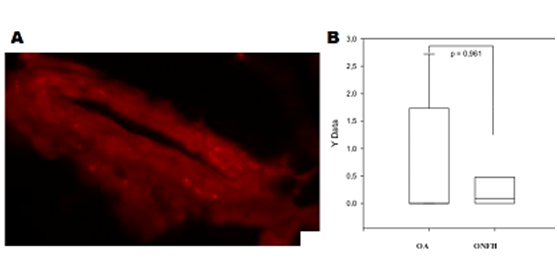
Figure 3 Sympathetic nerve fibers in the femur bone.
Figure 3A Sympathetic nerve fibers in the femur bone sample of a ONFH rabbit.
Figure 3B Comparison of the number of sympathetic nerve fibers per square millimeter between ONFH and OA femur bone samples (p = 0.036).
Figure 4A showed the sympathetic nerve fibers in the femoral head sample of an ONFH rabbit. No significant difference was found in the number of sympathetic nerve fibers per square millimeter between ONFH and OA samples from the femoral head, shown in Figure 4B.
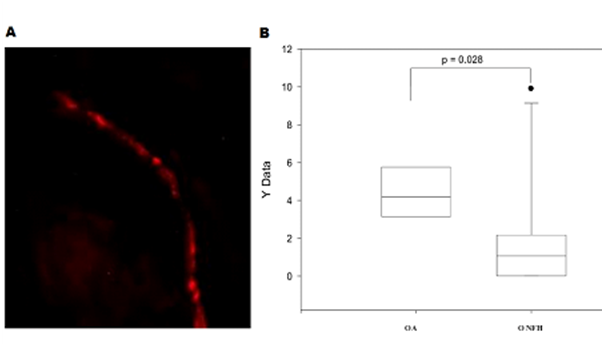
Figure 4 Sympathetic nerve fibers in the femoral head.
Figure 4A Sympathetic nerve fibers in the femoral head sample of an ONFH rabbit.
Figure 4B Comparison of the number of sympathetic nerve fibers per square millimeter between ONFH and OA femoral head samples (p = 0.961).
The samples of the ligamentum capitis femoris were also collected for both ONFH and OA rabbits. Figure 5A showed the sympathetic nerve fibers in the ligamentum capitis femoris sample of an ONFH rabbit. The number of the sympathetic nerve fibers per square millimeter showed a significant difference between ONFH and OA samples (p = 0.028), shown in Figure 5B. The significantly fewer sympathetic nerve fibers were observed in the ligamentum capitis femoris samples from ONFH rabbits than from OA rabbits.
At present, it is considered that the cause of ONFH is based on an altered hemodynamic. Atsumi et al. & Sevitt et al.17,18 found that there are no radiological necrosist onset, missing the vessels in the capsule of the upper femoral neck completely. It comes to hypoperfusion in the activation of an increased sensitivity of vessels to vasoconstrictors, and the body automatically tries to counter this lack effect by reducing the density of sympathetic nerve fibers .Since the ONFH patients complain groin pain, it seems likely that the number of sympathetic nerve fibers is increased. Findings on Sudeck's Atrophy (Reflex Sympathetic Dystrophy Syndrome) indicated that pain can be maintained by the sympathetic nervous system through a direct stimulation of nociceptive C fibers. Thus, the pain perception can be enhanced by injection of norepinephrine in patients with Sudeck's Atrophy.19 So, it was also suspected algodystrophy-like pathogenesis for ONFH.
However, the immunohistochemical analysis of various tissue samples showed that the sympathetic nerve fibers in the samples of OA rabbits were less than in that of ONFH rabbits. Significant differences in fiber density could only be found in the femur bone and ligamentum capitis femoris. A possible explanation of this fact can be explained by the fact that the body tries to respond to the disease. ONFH is demonstrably a slowly progressive degeneration of the hip bone, where it may take 80 months from initial diagnosis by MRI up to the onset of symptoms.14 So, the organism has time to react to these pathologies and to counteract. Because the blood flow seems to be defective, it is understandable that the body with a reduction in sympathetic nerve fibers attempt to improve circulation by dilating the blood vessels. It is now important to note that everything dies in the area of necrosis, even as sympathetic nerve fibers well as. Thus, it can explain why less sympathetic nerve fibers are present in the necrotic area, although this reduction is not significant compared to OA tissue.
Since in vitro experiments already successfully demonstrated that an inflammatory response to a reduction of sympathetic nerve fibers occurs, it seems likely that the origin of the necrosis is an inflammatory action.19,20 Straub et al.20 have shown that, number of sympathetic nerve fibers is reduced in synovial fluid of rheumatic joints.20 In the immediate environment of an inflammatory process, sensory nerve fibers are stimulated, and the hypothalamus-pituitary axis also stimulates the sympathetic nervous system. There is an increase in endogenous cortisol, adrenaline, noradrenaline and adenosine. Investigations by Straub et al.20 showed this effect on acute inflammatory reactions. When inflammation persists over a longer period, such as in rheumatoid arthritis, it seems to come to a kind of adaptation. The hypothalamus and the pituitary are not permanently active and decrease the endogenous steroid hormone and catecholamine.20 This reduction of corticosteroids and norepinephrine leads to the anti-inflammatory synergistic effect. The asthma therapy is known that both drugs act synergistically. Straub et al.21 showed that the serum levels of both cortisol and norepinephrine in rheumatoid arthritis patients are low, whereas the levels of IL-6 and TNF are elevated, suggesting a generalized inflammatory response.21 A bony inflammatory response would also explain why early stages of a scintigraphy can represent using technetium 99m mAb.4 Inflamed tissues have higher metabolic activity and thus enrich the administered nucleotides, so that the corresponding areas are visible scintigraphically. In the inflammation proceeds of necrosis, scintigraphy can be used in few months before the pathological X-ray.4 To confirm this, further laboratory tests are required in regards to the presence of inflammatory mediators and cells (eg. macrophages, granulocytes).
Drescher et al.22 has already managed to prove that the vessels reaction in necrotic tissue was changed under the influence of corticosteroids on the vasoconstrictors noradrenaline and endothelin-1 sensitivity.22,23 This poses the question whether the increased sensitivity is due to the influence of steroids, or even to the disease? It could be explained that the reduction of sympathetic nerve fibers might be a repair attempt of the organism. The SNS has long been recognized to play a central role in vascular regulation, and targeting sympathetic activity is certainly a therapeutical approach in the treatment of many vascular diseases. It is anticipated that assessment of both multiunit and single unit will remain central in future investigations related to vascular of ONFH. Here, it is considered almost certain that the tissue is hypersensitive to sympathetic activity. In summary, necrosis might be preceded by an inflammatory reaction, and an inflammatory response might lead to arthritic changes in tissue samples, which in turn reduced number of sympathetic nerve fibers.
In summary, necrosis might be preceded by an inflammatory reaction, and an inflammatory response might lead to arthritic changes in tissue samples, which in turn reduced number of sympathetic nerve fibers.
None.
None.

©2015 Xiaobing. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.