MOJ
eISSN: 2373-4442


Research Article Volume 4 Issue 5
1Department of Basic Research, Instituto Nacional de Oncolog
2Service of Medical Oncology, INOR, Cuba
3Universidad Aut
4Center of Molecular Immunology, Cuba
5Gynecology Service, INOR, Cuba
6Head Department of Basic Research, INOR, Cuba
Correspondence: Carlos Villegas Valverde, Immunologist, Laboratory of Immunology, Department of Basic Research, Instituto National de Oncología y Radiobiología (INOR), La Habana, Cuba, Tel +53-78388687
Received: January 01, 1971 | Published: December 9, 2016
Citation: Valverde CV, Miguel KL, Sánchez-Villanueva JA, Lambert AL, Portilla TC, Prado MCA (2016) Infiltration of Regulatory T Lymphocytes Impair Clinical Outcome in Ovarian Cancer Patients. MOJ Immunol 4(5): 00139. DOI: 10.15406/moji.2016.04.00139
Background: Ovarian carcinoma (OC) is one of the most severe and lethal cancers in women. Regulatory T (Treg) cells may participate in mediating a suppressive microenvironment in tumor tissue and contribute to tumor evasion from immune response. Specifically, some studies have indicated that Treg cell infiltration in malignant ascites and peripheral blood has been associated with poor clinical outcome.
Methods: The number of CD4+, CD25+, forkhead box p3+ (Foxp3+) lymphocytes was assessed via flow cytometry in ascites and peripheral blood from 24 women with epithelial ovarian cancer (EOC). Overall survival was associated with Treg levels.
Results: The study indicated an increase in the Treg cell subset from peripheral blood of EOC patients compared with those in healthy controls. Treg infiltration in ascites was higher than in blood into EOC patients. The patients with high infiltration of Treg in blood revealed less overall survival compared to a counterpart with normal levels of Treg. Regarding the association between patients with increased levels of Treg in ascites with overall survival there was no disadvantage found, compared to ascites from patients with normal levels of Treg.
Conclusion: Study suggests that Treg lymphocytes were increased in ascites and peripheral blood in EOC patients and could be associated with diminished overall survival, via impairment antitumor immune response.
Keywords: ovarian cancer, regulatory t cell, eoc, treg, overall survival, oc, patients, tumor immune cells
OC: Ovarian Carcinoma; EOC: Epithelial Ovarian Cancer; CTL: Cytotoxic T Lymphocytes; : Tumor Infiltrating Lymphocytes; BD: Becton Dickinson; GITR: Glucocorticoid Induced T Receptor; TIM3: T Cell Immunoglobulin Mucin 3; CTLA-4: Cytotoxic T-Lymphocyte Antigen-4
Ovarian cancer occupies the 6th place in incidence and mortality in woman on a worldwide scale and it is the most lethal of all gynecological cancers. In Cuba it occupies 5th place in incidence, among the female population between 18 and 45 years of age and is the seventh cause of cancer after 45 years of age. It is diagnosed at advanced disease (stage III/IV as defined by the International Federation of Gynecology and Obstetrics FIGO) in more than 75% of patients, and tends to progress in a clinically indolent way in more than half of its natural history. The risk of suffering a sporadic ovarian cancer in life is 1,7% and it increases from 10% to 40% if there is family history, for instance germline mutations in BRCA1 or BRCA2.1-4
Primary cytoreduction by surgical debulking, followed by platinum and taxane-based combination chemotherapy is the standard combination of treatment in this type of cancer. Nevertheless, in most of patients at diagnosis have hematologic, lymphatic and transperitoneal disseminations, in this condition the surgery is multiorganic and highly complex, reducing the survival possibilities.3,5 This therapeutic conduct in advanced stages promotes a prognosis up to 5-years survival rate lower than 30% of patients. In addition to this problem appears the platinum-resistant tumors, that represent about 50% of all cases, which impact in the survival rate can extend into 6 months after therapy is initiated.3,6
It has been observed that different women with ovarian cancer, of equal lineage, grade of differentiation, genetic background, FIGO stage, age, race and similar risks, show different response to the treatment and very different prognoses. Therefore, it was proved that they had differences in the tumor immune cells infiltration and it was possible that, on these characteristics, there was taking root the cause of the different destinations of these patients. In this sense, one of the components most studied in this type of illness is the regulatory T lymphocytes (Treg).7,8 In fact, the association between Treg and prognosis was made for the first time in ovarian cancer.9
The Treg can be characterized as an immunophenotype CD3+, CD4+, and Foxp3+. These cells are antigen specific and they are attracted to the tumor microenvironment from the circulation to accomplish theirs functions, but also they recirculate through the whole organism equally to the rest of the lymphocytes. Therefore, they can accumulate in tumor microenvironment, ascitic fluid associated with the tumor and blood, but with different levels due to movement driven by chemokines.10
These lymphocytes subpopulations are powerful regulators for activity of the other T cells such as cytotoxic T lymphocytes (CTL). The regulation is mediated by direct and indirect mechanisms, depending on increase of soluble factors such as the adenosine and different cytokines: IL-10, IL-35 and TGF-β or local sequestration of IL-2, as well as other driven for cell-cell contact: CTLA-4/CD80, CD86; PD1/PD1L, PD2L; among others. When CTL infiltrate the tumor and are functional, they are associated with the destruction of cancerous cells and are also associated with a decrease in tumor volume concomitant with a significant survival prolongation. Following this line of thought the Treg levels might affect the citotoxic response to the tumor and worsening the clinical outcome and facilitating cancer progression.10
The ascites in the advanced ovarian cancer is frequent and represent about 38% of all the causes of ascites in female patients.11 This malignant ascites is considered an extension of the tumor microenvironment. The characterization of cancerous cells and leucocytes, extracted from this fluid, can allow to confirm and complete the disease diagnosis and prognosis. Besides, it is less invasive test. Indeed, the transperitoneal way is used for the administration of antineoplastic and immunomodulators drugs, achieving a faster access to the tumor microenvironment.11 Consequently, the immune cells composition of the ascites in epithelial ovarian cancer should be an image of the tumor microenvironment.
The aim the current research was to evaluate the association between Treg infiltration in ascites and peripheral blood, with the clinical outcome of epithelial ovarian cancer (EOC) patients, treated at the National Institute of Oncology and Radiobiology of Cuba.
Sample
The patients included in the study came from the service of gynecology of the National Oncology and Radiobiology Institute of Cuba (INOR), diagnosed with EOC, classified as stage IIIC/IV according to FIGO. The diagnosis confirmation was made by histopathological analyze, validated by double test. The informed consent was obtained in writing form from all patients, following the international ethical norms for the achievement of research in human beings and the approval ones by the INOR. The human sample was taken from 24 women older than 18 years, no gravid, previously untreated without any specific cancer treatment and immunomodulator drugs. Controls come from female healthy volunteers by 27 women older than 18 years (Table 1).
|
Characteristics |
n |
Percentage |
|
|
Age |
≤ 50 years |
6 |
25,0 |
|
> 50 and ≤ 60 years |
5 |
20,8 |
|
|
> 60 and ≤ 70 years |
8 |
33,3 |
|
|
> 70 years |
5 |
20,8 |
|
|
Skin Color |
White |
14 |
58,3 |
|
Black |
6 |
25,0 |
|
|
Mixed |
4 |
16,7 |
|
|
FIGO Classification |
IIIC |
21 |
87,5 |
|
IV |
3 |
12,5 |
|
|
Histologic Type |
Unclassified Adenocarcinoma |
5 |
20,8 |
|
Papillary serous Adenocarcinoma |
9 |
37,5 |
|
|
Mucinous Adenocarcinoma and Cystadenocarcinoma |
7 |
29,2 |
|
|
Mixed Epithelial Malignant Tumor |
3 |
12,5 |
|
|
CA125 (n=18) |
≤ 500 U/ml |
7 |
35,0 |
|
> 500 U/ml |
11 |
55,0 |
|
Table 1 Clinical characteristics of the epithelial ovarian cancer patients (EOC) (n=24)
From these patients 25 ml of ascitic fluid were obtained by paracentesis during income, and they were discharged 10ml in 2 Vacutainer Becton Dickinson (BD) with EDTA. The peripheral blood was obtained via venipuncture and 10 ml were excused in 2 Vacutainer BD with EDTA. Both samples were processed for flow cytometry into 6 hours from collecting time.
The clinical information was obtained during clinical follow-up of the patients. The patients were followed-up for 33 months.
Flow cytometry
For flow cytometry the mononuclear cells were isolated from ascites and blood by gradient centrifugation (Histopaque, Sigma), mononuclear cells/ml concentration of 1x105 were obtained to process for staining. First surface clusters (CD4 and CD25) were labeled with specific monoclonal antibodies (mAbs), later the intracellular detection anti-Foxp3 was performed using fixed and permeabilized cells, according to the manufacturer’s instructions. Were used combined mAbs: anti CD4 phycoerythrin (PE), anti CD25 PerCp and anti Foxp3 Alexa Fluor 488, from Dako, Denmark. The cells were assessing with a cytometer FACscan (Becton Dickinson), 10000 events each sample were considered.
Statistical analyses
To assess the possible association between the variables was used X2 test of Independence, in addition to the Pearson Linear Coefficient Correlation was used for quantitative variables.
To establish comparisons between two groups for quantitative variables, Mann-Whitney for two independent samples, with One-tailed P values were used. To enable a comparison between blood and ascites in patients, a Paired t test, with One-tailed P values was used.
To estimate the overall survival rate we used the statistical method of Kaplan - Meier. Median survival times were analyzed using Kaplan-Meier methods. 95% confidence intervals (IC) were computed where possible. Differences in survival functions were assessed using the log rank test. In order to analize the global survival rate we took into account the time elapsed between the diagnosis date and the passing of the patient.
Differences were considered significant at p<0.05. The statistical package SPSS, version 20.0 for Windows was used.
Evaluation of treg levels (CD4+, CD25+, Foxp3+) in peripheral blood and ascites
The CD4+CD25+Foxp3+ cells cytometry were quantified in peripheral blood and ascites from patients with ovarian cancer (Figure 1a&1b). In the control group healthy women (n=27) used in this study, Treg concentrations were found between 0.12% and 2.20% with mean of 1.7% SD=0.51, which coincides with the international values for these cells.9,10,12
In accordance to this mean in the patients 2 groups were defined:
We first examined the frequency of CD4+CD25+Foxp3+ cells in ovarian patients and healthy controls. We found that the proportion of CD4+CD25+Foxp3+ cells in patients was elevated in both as blood as ascites with of 55% and 80% of the patients respectively (Figure 1c). The mean value of CD4+CD25+Foxp3+ cells in the patients’ blood, showed significant differences compared with the mean of the control sample: (2.7% vs 1.7% *p=0.036). In patients with high CD4+CD25+Foxp3+ cells levels (n=11) the mean value was 4,05%, (IC:95% 3.16-4.93) compared with healthy controls that shown 1.7% (IC:95% 3.16-4.93%) more significant (p <0.01). In the ascites CD4+CD25+Foxp3+ T cells were raised up to 80% with high levels, (3.3-10.31%), mean of 5.4±2.47%, with significant differences compared with published data. This study did not count with group control to determine values of normality in non-malignant ascites, by which the above mentioned values were obtained from literature which establishes a status of 0.7-5.0% of CD4+CD25+Foxp3+ cells. 3.2% was established as cutoff level for the determination of high CD4+CD25+Foxp3+ cells accumulation.9
Having compared the means of the values of the CD4+CD25+Foxp3+ cells between both studied samples, the result was statistically significant, according to test U of Mann-Whitney with ***p=0.0006 (Figure 1d).
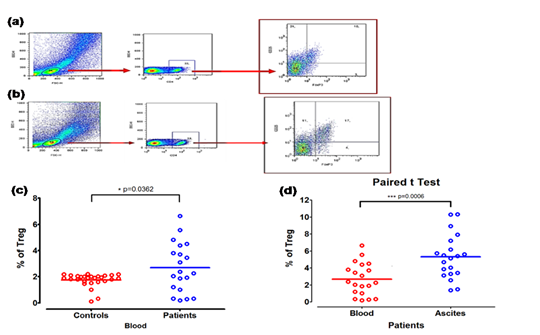
Figure 1 Frequency of Treg in blood and ascites samples. (a) Gating strategy for CD4+CD25+Foxp3+ Treg in blood, greater than 10000 events per gate was assessed. (b) Gating strategy for CD4+CD25+Foxp3+ Treg in malignant ascites, greater than 10000 events per gate were assessed. (c) Comparison Treg’s percentage in healthy controls blood and patients’ blood (n=20) (*p=0,0362). (d) Percentage of Treg subset. (n=20) ***p=0.0006, compared with Mann-Whitney U test, within blood and ascites from patients.
Assessment of the association between the levels of CD4+CD25+Foxp3+ cells and the patient’s survival
It is very relevant for oncological clinic practice to know the different factors that could be associated with the overall survival in the patients with ovarian cancer. The assessment of the association between the Treg levels in blood and the overall survival, was performed by means Kaplan – Meier estimate. In the patients with raised levels of Treg in blood (Figure 2 & Table 2) the survival median was 10 months, practically half that was found for healthy controls, who showed an average of 18 months (n=20) (p=0.0968). Although these results were not statistically significant, a tendency was demonstrated to the reduction of the overall survival in the patients with high Treg levels in peripheral blood. The analysis of the survival means exhibited an equal tendency (17 vs 9 months, Table 2).
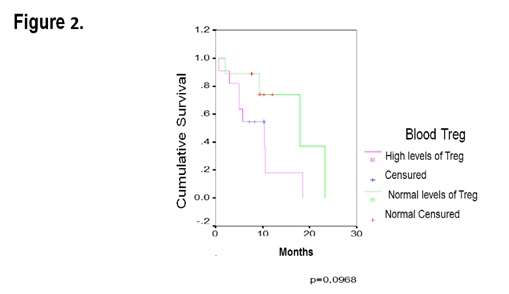
Figure 2 ThreeKaplan-Meier curve for overall survival by levels of blood Treg cells in 20 patients with EOC.
|
Treg Levels |
n |
Median |
CI 95% |
Mean |
CI 95% |
Long Rank Test |
|
Normal |
9 |
18 |
6 – 30 |
17 |
11 - 23 |
0,0968 |
|
High |
11 |
10 |
3 – 17 |
9 |
5 - 13 |
Table 2 Median and mean of overall survival of the EOC patients, with 95% of IC (Blood)
Regarding the association, the ascites CD4+CD25+Foxp3+ cells levels with the overall survival, we got a different result from what we expected but these were not statistically relevant (n=20) (p=0.5458). Both groups of patients had a median of survival of 10 months. Hence, in the studied sample survival advantage did not exist for patients with high Treg or with the normal levels in the malignant ascites (Figure 3 & Table 3).
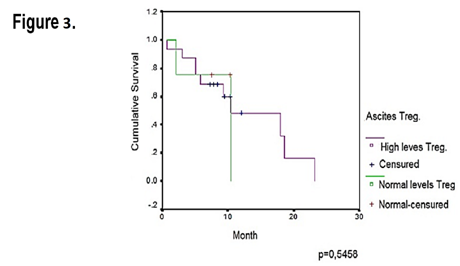
Figure 3 Kaplan-Meier curve for overall survival by levels of ascites infiltrating Treg cells in 20 EOC patients.
|
Treg Levels |
n |
Median |
CI 95% |
Mean |
CI 95% |
Long Rank Test |
|
Normal |
9 |
10,56 |
3,33 - 13,54 |
8,44 |
3,33 - 13,54 |
0,5458 |
|
High |
11 |
10,40 |
8,57 - 17,14 |
12,86 |
1,47 – 19,33 |
Table 3 Median and mean of overall survival of the EOC patients with 95% of IC (Ascites)
In accordance with the physiological function of cells with the phenotype CD4+CD25+Foxp3+, one expectations from these cells is, when they increase in the tumor microenvironment they become a part of the evasion mechanisms of the antitumor immune response.11 The tumor leakage to the immune system constitutes one of the Hallmarks of the cancer described by Hanahan and Wierberg.13 Thus, the increase of the Treg in the environment of the EOC, as well as in the ascites are associated with bad prognosis and generate a negative impact in the overall survival.8,9,14
The expression of the master regulator Foxp3 in these lymphocytes, it’s related to the suppressive function on effectors lymphocytes populations, like the CTL Tumor infiltrating lymphocytes (TIL) inside tumor that destroy cancer cells. Several studies had established that the mere measurement of the overexpression of this transcriptional factor, it’s associated significantly with the reduction of the survival in the ovarian cancer patients. This transcriptional factor modulates the positive expression of several molecules such as, CD25, alpha chain of the high affinity receptor for the IL-2, Glucocorticoid Induced T receptor (GITR), T cell immunoglobulin mucin 3 (TIM3), cytotoxic T-lymphocyte antigen-4 (CTLA-4) and the couple programmed cell death protein 1 (PD‑1) and its ligand PDL-1 and PDL-2.9,10,12,14,15
The above mentioned molecules are very involved in the phase of immune contraction of the response and in the functional depletion (clonal specific) of the effectors lymphocytes. In fact, there are checkpoints, very sensitive, that are studied very intensely nowadays as therapeutic targets, with great positive impact in the reversal of the immunity suppression in the patients with cancer and a significant increase of survival.16,17
The increase of the Treg in both compartments (ascites and blood), according to previous ovarian cancer studies, is the result of the recruitment through chemokines generated by the tumor microenvironment cells, the interconversion of T helper patterns and by the induction of the T regulator pattern inductible in situ.9,18
In the tumor tissue there are cells, such as: tumor associated macrophages that secrete the chemokine CCL22 that which cause the recruitment of Treg cells to the tumor microenvironment via the CCR4 receptor. At the same time, the grade of hypoxia in the tissue provokes the liberation of CCL28 that also attracts Treg to the tumor microenvironment. The negative regulation of selectin ligand CD62L and the receptor CCR7 in the Treg, prevents migration to the lymph nodes and retained in the tumor microenvironment, also contribute moreover to its predominance in the tumor. In the same sense, the ascites contains similar composition, which is considered to be an extension of the tumor microenvironment, while in blood the Treg elevation is due to TGF-β liberation precisely for the Treg from the tumor tissues. This growth factor guarantees the maintenance of this T helper pattern and simultaneously it induces lymphocytes differentiation T virgin to Treg.9,14
Depending on how good the structural organization of the suppressive cells of the immune system are, within the tumor microenvironment, the better the systemic repercussion will be and more Treg will be able to be recruited towards the tumor. Nevertheless, the Treg concentration in the blood will be relatively less because the cells remain for less time and are distributed in a much bigger volume in this compartment, as well as the migration directed by chemokines drive the lymphocytes traffic from the blood to the tumor tissues and lymph node where the immune response is induced.9,14,19
The association was demonstrated between the high Treg levels in blood and the overall survival, although it was not significant. These cells due to their functions not only suppress the antitumoral cytotoxic activities of several cellular populations such as: NK, CTL, Tγδ, etc. However, by means of the production of TGF-β and IL-10 conducive more remodeled of the microenvironment of the tumor and increase of suppressive cells levels that perpetuate not resolve inflammation and increase the fluids collection in the peritoneal cavity.11,19,20
The tumor proliferation maintained and facilitated by the suppress function of the Treg, the increased consumption of nutrients and energy by the metabolic epithelial and stromal activity with enormous participation of immune system cells that also they have a high proliferation rate, and the effect of mass of the ascites per se and tumor is contributed by them to the reduction of the survival of the patients. Ascites can cause debilitating symptoms as a substantial volume of fluid can cause pain, early satiety and respiratory compromise. The gastrointestinal and urinary systems can also be affected, resulting in significant morbidity. The Treg are a part of a scenario that favours the growth and progression of the tumor.11,21-23
In this research the correlation between ascites Treg and overall survival, there were not differences between both groups defined according to Treg levels. This could be due to the fact that these cells are not the only ones involved in the suppressive inflammatory infiltrator. There are several populations demonstrated at present that show similar behavior: suppressive cells derived from myeloid, immature dendritic cells, TAM with profile M2, neutrophils N2, etc. The organization of the Treg in structures as tertiary lymphoid organs and its preferential location whether intraepithelial or stromal also it influences the magnitude and aftereffect of its functions. Studies had suggested that the stromal play a domineering role in the recruiting of more Treg and other suppressive cells. In this study, which has only been done in the ascites, it was not possible to evaluate the histological Treg distribution in the tumor.11,20-22
Another population in which there were also negative effects during the illness were CD8+ Treg cells, described in the ovarian cancer and that can have synergic action with its pairs CD4+. Idem that occur with CD4+ Treg, in the case of Treg CD8+ it is not enough to know if they are high in the tumor, it is necessary to see in what tumor area they have high representation, whether intraepithelial or stromal; since its negative effects on the overall survival differ according to its location is preponderant.22
This complex scenario determines eventually the clinical outcome of the illness and can exhibit peculiarities in every patient, therefore relating just one cellular population to the patient´s clinical fate is a bit reductionist and less desirable nowadays. It becomes necessary to quantify cells and to measure their functionality to be able to predict better its impact in the prognosis, departing from the proper immunoclassification.
The based evidence across this study, it suggests that the increase of Treg cells in ascites and peripheral blood in patients with advanced ovarian cancer, result in a negative impact in the overall survival. The assessment of specific populations allows to identify the magnitude of its role in the immunopathogenesis of the ovarian cancer. Nevertheless, the integral approach to identify pattern or signatures, where there are evaluated the components of the immune system infiltration, will be decisive for patients immunoestage and to predict the most individualized and accurate prognosis in these kind of patients.
None.
None.

©2016 Valverde, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.