MOJ
eISSN: 2373-4442


Research Article Volume 2 Issue 3
1Department of Biochemistry, Ladoke Akintola University of Technology, Nigeria
2Department of Biochemistry, Federal University of Technology, Nigeria
Correspondence: Olawoye TL, Department of Biochemistry, Federal University of Technology, PMB 704, Akure, Nigeria. Tel: +234(0)8033601166,
Received: August 01, 2015 | Published: August 18, 2015
Citation: Adedeji AL, Olawoye TL (2015) Elevated Serum Alpha-1-Antitrypsin Concentration is Associated with HIV Disease Non-Progression. MOJ Immunol 2(3): 00046. DOI: 10.15406/moji.2015.02.00046
Association between reduced serum alpha-1 antitrypsin (α1AT) concentration and HIV infection has been reported. The possible role of α1AT in a subset of people living with HIV who achieved control over HIV-1 disease progression without treatment has not been substantiated. We thus hypothesized that increased serum concentrations of α1AT would be associated with control of HIV disease progression in the absence of highly antiretroviral therapy (HAART). We compared serum concentrations of α1AT in a cross-section of HIV-1-infected subjects naïve to HAART and yet resist progression to AIDS (HAART-) with those in whom control of disease progression was achieved by HAART (HAART+). Mean α1AT concentration was significantly higher in the HAART-group (177±17mg/100mL) compared with HAART+ (126±12 mg/100mL; p=0.018). Serum α1AT concentrations less than 100mg/100mL were found in 14% and 41% in HAART- and HAART+ group, respectively. Serum α1ATconcentration and blood CD4+ T cells count in the HAART- group were positive correlated (R=0.426; p=0.021). Increased expression of α1AT may contribute to effective control of HIV disease progression in the absence of HAART. Our findings support the possible use of alpha-1-antitrypsin in HIV disease management.
Keywords: alpha-1-antitrypsin, HIV disease non-progression
Alpha 1-antitrypsin (α1AT) is a glycoprotein, synthesized majorly in the liver and to lesser degree in extra-hepatic tissues.1 In normal adult populations, the 95% reference range in blood is 100-270 mg/100ml.2 Its serum concentration can raise many folds during acute inflammation.3 The main physiological function of α1AT is inactivation of potentially harmful proteinase, notably neutrophil elastase.4,5 The interaction between a typical proteinase and α1AT is non-covalent. The bound complex renders the proteinase and α1AT inactive. While the neutrophil elastase is completely inactive in the presence of α1AT, the cleaved peptide of α1AT is still immunologically active. The cleaved peptide has been demonstrated to have neutrophil chemo-attractant properties.6 This phenomenon emphasizes the importance of α1AT in inflammatory reactions.
Furthermore, there are evidences that α1AT exerts antimicrobial activities. Knappstein et al.7 reported that α1AT binds to the secreted enteropathogenic E. coli proteins and strongly reduces their mediated haemolysis of red blood cells. Bilello et al.8 also showed that α1AT possesses anti HIV activities in vitro. Since α1AT neutralizes other proteases, the functions of proteases required for HIV-1 propagation may also be inhibited. Functional levels of α1AT may therefore be associated with control of HIV disease progression. Although, association between reduced serum α1ATconcentration and HIV infection has been shown to be consistent with a role for α1ATas an endogenous HIV suppressor,9–11 it is not very clear whether α1ATplays significant role in subsets of people living with HIV who achieved control over HIV-1 disease progression without treatment. We thus hypothesized that increased serum concentrations of α1ATwould be associated patients who exhibit natural control of HIV disease progression in the absence of highly active antiretroviral therapy (HAART-). In the present study, we compared serum concentrations of α1ATin subset of HIV-1-infected subjects naïve treatment and yet resist progression to AIDS with subjects whose control of disease progression was achieved by HAART as this may reveal the possible use of α1AT in the management of HIV disease.
Selection of subjects
A cross-section of sixty-eight HIV-1-infected subjects attending Living Hope Care (LIHOC), Ilesa, Nigeria was studied. LIHOC is a Non-Governmental Organization, providing care for people living with HIV/AIDS. Status and demographic characteristics and antiretroviral therapy records was collected. Thirty-three of subjects had been receiving effective HAART [Lamivudine (300 mg/day), Stavudine (60 mg/day) and Nevirapine (400 mg/day)]. Twenty-nine subjects had not received any antiretroviral therapy as at the time of enrolment. Six subjects who exhibited clear evidence of acquired immune deficiency syndrome (AIDS) were also included. Twelve healthy HIV-1 sero-negative volunteers were recruited within the vicinity of the centre for this study. Volunteers with additional conditions that could affect evaluated parameters, such as pregnancy and tuberculosis infection were excluded from the study. Informed consent was obtained from all volunteers before initiation of the study. The LIHOC Ethical Committee of approved the study.
Specimens collection and preservation
Blood sampling was done by venipuncture and transported under ice-cold condition to the laboratory within six hours. Serum was separated from whole blood by centrifugation at 1000 x g for ten minutes and stored in aliquots at -20 0C until analyzed.
Diagnosis of HIV infection and CD4 T-cell cytometry
The diagnosis of HIV-1 infection was performed by enzyme link immunosorbence assay (ELISA) and confirmed by HIV Western Blot using ImmuneticsQualicode HIV-1/2 Kit (USA). Subjects with indeterminate results were excluded from the study. Control subjects were also confirmed to be negative for antibodies to HIV.EDTA-anticougulated blood CD4T-cell was enumerated using Cyflow® Cytometer according to the manufacturer’s instructions (Partec, Germany).
Alpha 1 antitrypsin (α1AT) assay
The serum concentration of α1AT of subjects was determined using a commercial radial immunodifussion assay plates purchased from Liofilchemsrl, Italy. The procedure was carried out according to manufacturer’s instructions.
Statistical analysis
Descriptive analysis, and student t-test and Spearman correlation were respectively, employed to compare data and test association between variables using Graph Pad 5 software (San Diego, CA). P-values <0.05 were considered significant.
Characteristics of study subjects
HIV-1 infected subjects naïve to HAART had a median CD4 lymphocyte count of 440 (IQR 321-510) cells/µl while that of HIV-1 infected subjects under HAART was 410 (IQR 300-605) cells/µl. Although, the male to female ratio was approximately 0.3, the blood CD4 T-cell counts of the groups were not significantly different (p=0.527). The detailed characteristics of the subjects are shown in Table 1.
Characteristics |
HIV- |
HAART- |
HAART+ |
HAART+/AIDS |
p-Value* |
N |
12 |
29 |
33 |
6 |
|
Age (year) |
37 (35, 45) |
35 (34, 49) |
35 (32, 47) |
42 (31, 61) |
0.271 |
MAC(cm) |
28 (24, 29) |
28 (26, 30) |
27 (27, 31) |
20 (19, 24) |
0.6 |
Sex (M/F) |
02-Jan |
22-Jul |
26-Jul |
03-Jan |
0.405 |
ID (month) |
NA |
12 (4, 44) |
16 (10, 55) |
13 (7, 19) |
0.236 |
HD(month) |
NA |
0 |
14 (5, 48) |
13 (7, 19) |
NA |
CD4 count (/μl) |
734(634, 825) |
441 (319, 511) |
410 (300, 600) |
230 (165, 295) |
0.527 |
Table 1 Characteristics of study subjects
Values are medium (25th and 75th percentile). p-values were determined by Student’s ‘t’ test and Fisher’s exact test, as appropriate to compare HAART- and HAART+, p <0.05 was considered significantly different. NA, not applicable; MAC, mid arm circumference; ID, infection duration; HD, HAART duration
Serum alpha-1-antitrypsin (α1AT) concentration under HIV-1 disease non-progression
The concentration of α1AT in the serum of HIV-1 infected subjects under HAART (HAART+) was compared with those naïve to HAART (HAART-). The HAART- group had a significantly elevated serum concentration of α1AT than the HAART+ and HIV-1 uninfected control group (p=0.018 and p=0.015, respectively Figure 1s. Serum α1AT concentrations <100mg/100mL were found in 14% and 41% in HAART- and HAART+ group, respectively. Under effective HAART+, subjects with CD4+ T cell counts >500 cells/µl had similar serum concentration of α1AT with those having CD4+T cell counts <500 cells/µl (p=0.696). However, the HAART- group with better CD4+ T cell counts had higher concentration of α1AT, although this was not significant(p=0.142) Figure 2. Treatment duration did not have significant effect on the expression of α1ATin the serum (p=0.442) Figure 3.
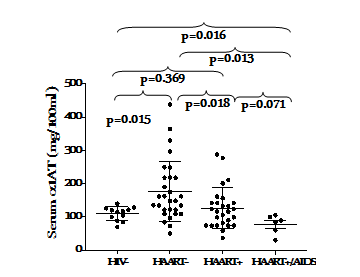
Figure 1 Serum alpha-1 antitrypsin concentration under effective control of HIV disease progression. The dots and error bar represent individual measurements and mean with SD, respectively. P-values were determined by Student’s t test and are shown for comparison. p< 0.05 were considered significantly different.
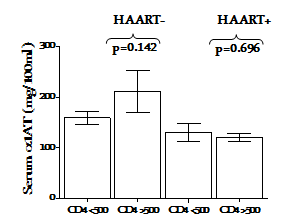
Figure 2 Impact of HIV clinical status on serum alpha-1 antitrypsin concentration under effective control of HIV-1 disease progression. The bar and error bar represent mean ± SEM. p-values were determined by Student’s t test and are shown for comparison. p<0.05 were considered significantly different.
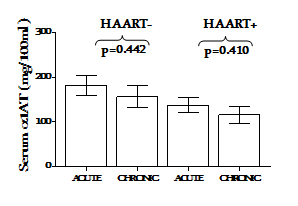
Figure 3 Impact of HAART/infection durations on serum alpha-1 antitrypsin concentration under effective control of HIV-1 disease progression. The bar and error bar represent mean ± SEM. p-values were determined by Student’s t test and are shown for comparison. p< 0.05 were considered significantly different.
Association between serum alpha-1 antitrypsin (α1AT) and marker of HIV disease status
The concentrations of individual serum α1ATin subjects infected with HIV-1 in both HAART+ and HAART- groups in relation to their blood CD4 counts were correlated. A significant positive correlation was found between serum α1AT and blood CD4+ T cells count in the HAART naïve group (R=0.426; p=0.021) and no significant correlation was observed in the subjects under HAART (R=-0.136; p=0.500) Figure 4.
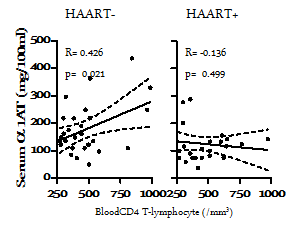
Figure 4 Association between serum alpha-1 antitrypsin (α1AT) and marker of HIV disease status (blood CD4 counts) under effective control of HIV-1 disease progression. Each dot represents an individual subject’s α1AT concentration. Regression lines and Pearson R-values are shown for correlations. The dotted lines are 95% confidence band. p-value< 0.05 were considered significant.
Alpha-1-antitrypsin (α1AT) has been identified as the most abundant endogenous protease inhibitor, also found to inhibit human immunodeficiency virus (HIV) replication.3,12–14 We hypothesized that HIV-1 infected subjects that resist progression without treatment (HAART-) wound exhibit a higher serum levels of α1AT than in subjects in whom control of disease progression was achieved by HAART. It was first investigated whether increased serum concentration of α1AT would is associated with effective control of HIV-1 infection in the absence of HAART and then whether serum α1AT concentrations were associated with clinical (CD4 counts) status.
It was discovered that the serum concentration of α1AT was higher in HIV-1 infection under effective control of disease progression than in HIV-1 uninfected subjects. This is not in complete agreement with Bryan et al.10 who reported that HIV infection was associated with reduced concentration of α1AT compared with HIV-1 uninfected control In our present study, only a cohort of HIV-1-infected subjects with evidence of AIDS exhibited reduced concentration of α1AT compared with HIV-1 uninfected control (p=0.016). The discrepancies might be attributed to the fact that the majority of HIV-1 infected in Bryan et al.10 study met the 1993 CDC criteria for AIDS15 while only six subjects in our present study met the AIDS criteria. Evidently, HIV disease progression was well controlled in our subjects; either by host immunological responses (HAART-) or by ARV (HAART+). It is also interesting to note that, compared with HIV-uninfected control, significantly (p=0.013) low levels α1AT was found in subjects with evidence of AIDS in our study. We are not certain whether the subjects that resist progression to AIDS in the absence of treatment constitutively expressed elevated α1AT prior to infection or the increased expression was a natural response to HIV infection in the cohorts. In a previous study, Adedeji et al.16 showed that this group of subject possessed and retained the ability to synthesized immunoglobulin. It is also possible that the cohort possessed the natural ability to respond to HIV infection by synthesizing α1AT and of course, increased expression α1AT would contribute to non-progression in the absence of treatment. Normal α1AT phenotype may also be associated with the ability of host to express the protein as abnormal α1AT phenotype has been demonstrated to be associated with HIV disease progression2 and HIV infection in a patient with alpha-1 antitrypsin deficiency has been described as a detrimental combination.17 However, Ferreira et al.,18 suggested that deficiency in α1AT may be a risk factor for acquisition of HIV infection, but physiological α1AT concentrations do not affect disease progression after infection occurs.
Based on treatment status, HIV-1 infected subjects that resist progression without treatment (HAART-) exhibited a significantly (p=0.018) higher serum levels of α1AT compared to subjects in whom control of disease progression was achieved by HAART (HAART+).α1AT is an acute phase protein with HIV-1 inhibitory properties13 and tissues inhibitor of serine proteinase implicated in the regulation of inflammation and host defense,19,20 the elevated α1AT may be associated with control of HIV replication and thus disease progression in the absence of HAART. Although Bryan et al.,10 reported no association between serum α1AT levels and viral load or use of antiretroviral therapy in HIV-infected subjects; we were unable to determine the viral load to compare the extent of viral replication control more importantly in the HAART-naïve group with elevated serum α1AT concentration. Further studies will focus on the association between of elevated serum α1AT concentration and viral load in HIV-infected subject who resist progression in the absence of HAART.
Stratification based on HIV clinical status and HAART/infection duration added another dimensions to the present study. We compared the α1AT concentration in groups defined by the blood CD4 count (CD4 <500 or >500/µl). While no apparent difference was observed in the in HIV-1-infected subjects under HAART (p=0.696), subjects with better clinical status exhibited a higher (although not statistically significant p=0.142) α1AT under HAART Figure 2. This indicates the possible association of elevated α1AT concentration with clinical status. Bryan et al.10 found no association between very low α1AT concentrations and clinical status. It is interesting that we found a significant positive correlation(R=0.426; p=0.021) of α1AT with marker of disease status in subject who resisted progression in the absence of HAART Figure 4. We similarly compared serum α1AT concentration in groups defined by infection and HAART duration (Acute or chronic). In this study, we employ our earlier criteria16 to classify the subjects. Although, increased better outcomes had been associated with longer HAART duration, especially in preventing mother to child transmission,21 serum α1AT was not associated with HAART duration in the present study Figure 3.
Summarily, we have shown that host ability to exhibit increased expression ofα1AT is associated with HIV-disease non-progression in the absence of HAART. Our findings support the possible use of alpha-1-antitrypsin in HIV disease management.
We conclude that significantly higher serum α1ATconcentration contributes to the effective control of HIV-1 disease progression in the absence of HAART. Determination of α1AT phenotypes of this subset may provide more insight into this phenomenon.
We are grateful to the support group members, in Ilesa, Iwo and Ejigbo for participating in this study. Support of LIHOC staff members is gratefully appreciated. We acknowledge the financial support from Federal University of Technology, Akure (FUTA) Senate Research Grants (URC/MAJOR/168).
The authors declare that there have no conflicts of interest.
None.

©2015 Adedeji, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.