MOJ
eISSN: 2373-4442


Research Article Volume 6 Issue 5
University of Zimbabwe, Zimbabwe
Correspondence: Judith Musona Rukweza , University of Zimbabwe, P O Box A 198. Mazoe street. Avondale Harare, Zimbabwe,, Tel 00263773917910
Received: January 01, 1971 | Published: November 15, 2018
Citation: Rukweza JM. Effectiveness of screening and treatment for asymptomatic bacteriuria in reducing preterm birth: a systematic review of randomized controlled trials. MOJ Immunol. 2018;6(5):143?149 DOI: 10.15406/moji.2018.06.00210
Asymptomatic bacteriuria is a common infection among pregnant women. If untreated it often progresses to pyelonephritis later in pregnancy. Pyelonephritis is associated with pregnancy complications including low birth weight, stillbirth and preterm birth. Early detection and treatment of this infection could be effective in prevention of the complications. This systematic review was conducted to evaluate effectiveness of screening and treatment of asymptomatic bacteriuria during pregnancy in reducing preterm birth. Included were all randomized controlled trials with pregnant women as participants, comparators of control group/s and outcome of preterm birth. Excluded were all studies which did not use the randomized controlled trial and without control group/s. Search for articles was done electronically in databases including the PubMed, Medline, Science Direct, Google Scholar, HINARI, journal citation reports and trial registers. Study characteristics, bias and outcome analysis were evaluated. Out of 46 publications identified, only 2 trials were eligible for this review. One trial was done in 1962 and the other in 2015. The trials included 387 pregnant women out of 5462 recruited. There were conflicting results, with one trial reporting a significant reduction in preterm birth and no significant difference in preterm births following screening and treatment for asymptomatic bacteriuria. There is no convincing evidence on effectiveness of screening and treatment of asymptomatic bacteriuria in reduction of preterm birth.
Keywords screening, effectiveness, asymptomatic bacteriuria, preterm birth, systematic review, pregnancy, globally incidence, pyelonephritis, bacteriuria, placebo
Asymptomatic bacteriuria (ASB) is a condition in which a person without symptoms of urinary tract infection grows significant (>105 colony forming units per millilitre) and actively multiplying bacteria in a clean catch mid- stream urine sample.1The disease affect majority of pregnant women due to anatomical, mechanical and metabolic changes that occur during pregnancy.2 Globally incidence for asymptomatic bacteriuria among pregnant women range from 2.5% to 10% .3 Treatment of ASB generally lowers the risk of the infection developing to a symptomatic infection, pyelonephritis.3If untreated, ASB is associated with a 20% – 40% risk of developing an acute upper urinary tract infection (UTI), pyelonephritis occurring especially in the second or third trimester.4,5 ASB is also associated with PB 5. When pyelonephritis occurs during pregnancy there is high risk of delivering baby before due time, low birth weight and stillbirth among other adverse pregnancy outcomes.4 Screening and treatment of ASB is recommended.7 However, the role of screening and treatment of ASB in pregnancy in reducing PB is not clear. The purpose of this review was to synthesize evidence based on clinical trials findings on the role of screening and treatment of ASB in reducing PB. The findings are expected to help health care providers keep abreast with available evidence for policy makers to judge risks, benefits and harms of an intervention of screening of urine of pregnant women for asymptomatic bacteriuria. The findings are expected to expose gaps and influence more research in the subject of concern.
Review Question
What is the effectiveness of screening and treating asymptomatic bacteriuria during pregnancy in reducing preterm birth?
The protocol for this review was registered by PROSPERO, registration number CRD 42017067833. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was used to guide the preparation and conduction of this review.8
Inclusion criteria
This review included randomised controlled trials with pregnant women as participants being screened for ASB for treatment of those found positive for the disease and with a comparison control group to measure the difference between the two groups on PB incidence was considered eligible for this review. Preterm birth referred to birth of a baby before 37 complete weeks gestation calculated from first date of last menstrual period with or without an early scan. The frequency of screening intervention was not limited. There was no restriction on screening test used. There was no limitation on antibiotic selected for treatment. There was also no limitation as to type of preterm birth whether spontaneous or non-spontaneous. We considered all published articles both English and non- English, fitting the eligibility criteria.
Exclusion criteria
Excluded in this review were randomized controlled trial studies with no comparison group/s. Comparators were considered to be placebo, standard and routine treatment. Cluster randomized trials, cohorts, case control, cross sectional and qualitative studies were not considered for this review. Unpublished articles and studies that did not calculate gestation using last menstrual period and or a scan were also excluded in this review.
Information sources
The studies used in this review were identified by searching electronic databases. Both English and non- English papers were reviewed. The search was applied to electronic databases including MEDLINE, MEDSCAPE, Google Scholar, Google search, Research gate, and PubMed and HINARI. Manual search of referenced articles in the University library was also done. The search commenced on 03 April 2017. Reference lists of articles and abstracts were identified and used for this review. University library personnel were approached to assist with downloading of inaccessible articles. Registered clinical trials in trial registries were also used in the identification of some relevant articles.
Search and data abstraction
Data were abstracted for key variables including participant characteristics, intervention of interest, comparison or control group, outcome of interest and the study design. Basic search for information resources for this review was done through identification of key concepts including ‘screening for asymptomatic bacteriuria during pregnancy and preterm birth’, Randomised controlled trials on screening asymptomatic bacteriuria for preterm birth’, screening asymptomatic bacteriuria during pregnancy for reducing preterm birth’, preterm birth prevention strategies’. Key words including screening, asymptomatic bacteriuria, bacteriuria, pregnancy, pyelonephritis, preterm birth were selected for search of necessary resources. Synonyms and related terms for title elements were identified and used to search for relevant studies in the databases, for example premature delivery for preterm birth and urinary tract infection. Spelling differences, terminology and phrases for different languages were used as search terms. Truncations were used in phrase check and variable checks by using asterisk or question mark. Single, double or hyphens were used to search a phrase. Proximity operators were also used to create more complex single search. Field label was used to limit search to specific articles considering year of publication and title and abstract. All these strategies were used singly or in combination for a more complex search. The search was done electronically in databases including the PubMed, Medline, Science Direct, Google Scholar, HINARI, and Journal citation reports and trial registers. Relevant dictionaries, encyclopedias and key texts were used to search for alternative terms. The primary outcome for this review was preterm birth or gestation at time of delivery which may be term, preterm birth or post-dates.
Study selection
All studies fitting in the eligibility criteria were selected for this review. There was no limit to time or year of study and publication so as to reduce bias. Studies which included a comparison on impact on PB between screening and treating and not screening and not treating ASB during pregnancy were selected. Studies were considered eligible following reading of the title and methodology of abstract. The selected studies considered to be suitable for this review were printed and reviewed for consideration. Studies were selected from different selected databases. The search strategy described above was applied to identify the relevant studies for this review.
Data collection process
All review authors randomly selected relevant studies that met the inclusion criteria. One review author extracted data from the selected review articles. The other authors checked the extracted data that they critiqued, presented and summarized in this review. Some authors of the extracted articles were contacted for clarifying unclearly reported data. Authors were contacted to explain steps taken to reduce bias during data collection. Steps were taken to avoid extraction of data from similar reports by noting author, year of publication, sample size and outcomes of the studies. Information including individual characteristics, method of diagnosis, and inclusion and exclusion criteria of trials, type of intervention and inclusion of control group, and type of outcome measure were extracted for each trial.
Assessment of risk of bias in individual studies
Adequacy of randomization was assessed and where it was not clear in the report, the authors were contacted for clarification. Allocation concealment was considered for reducing bias. Blinding was also assessed for the participants, researchers, health care providers, data collectors, and outcome assessors. Single, double and no blinding was considered in this review. Extend of loss to follow up was assessed. This was done to ascertain the true effect of the intervention. The authors were emailed to clarify the methods they used to reduce bias. Summary measures. In this review the primary outcome of interest was PB which was obtained and calculated from dates of last menstrual period or ultrasound scan. Both statistical and clinical measures were used to assess effect of intervention. The outcome was summarized using odds ratio, relative risk and risk differences. Standardized mean difference also known as effect size was also used to standardize results of different studies.
Synthesis of results
Data extracted from the reviewed articles was processed before analysis. Results of individual studies included in this review were descriptively reported and analyzed using text and tables. Certain differences were reported by describing findings from individual studies.
Risk of bias across studies
To reduce possibility of bias across studies, all available studies that met the inclusion criteria were reviewed. Studies with significant and non-significant results were considered for review to prevent bias from missing studies. Missing studies were identified from trial registries. All studies which measure the outcome of interest in this review were considered in this review.
Out of 46 reports that were identified through the search strategies, 2 clinical trials were eligible for this review. A total of 387 (7.1%) participants participated in the trials out of the 5462 recruited pregnant women. Both the included studies for this review were randomized controlled trials. They were all published in English. Study selection. The flow diagram below illustrates study selection to report the total number of records that were identified in the electronic records. All the studies reviewed basing on inclusion criteria are shown on the flow diagram on Figure 1.
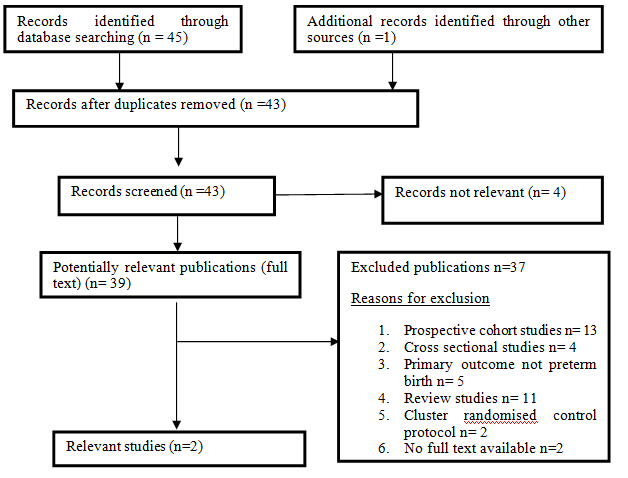
Figure 1 N=57; Epidemiological distribution of the pathological fractures, traumatic fractures, and nonunion.
Study characteristics
The research design, follow up periods, inclusion and exclusion criteria and the citation for the source of the selected articles are reported. Participants’ characteristics are also reported considering their age ranges3 and status. The intervention received was described for each study as well as the comparators. The primary outcome only was reported for each study. The main characteristics of the 2 included studies are presented in Table 1.
Authors |
Year |
Design |
Sample size (intervention/ control) |
Inclusion/ exclusion criteria |
Intervention/ comparator |
Setting & recruitment period |
Randomization process/ Follow up period |
Kass E.H |
1962 |
Randomized controlled trial |
1179 pregnant women, 1000 negative for bacteriruia |
Included were pregnant women presenting at first visit for antenatal care that had no bacteriuria symptoms, with less than 8 months of pregnancy. |
Treatment of bacteriuria with long acting sulphonamides followed by weekly or biweekly colony counts of urine until term. The comparator imvolved placebo treatment. |
Boston city hospital, Massachusetts,1961 |
All women with less than 8 months who tested positive for bacteriuria were treated. |
179 positive for bacteriuria |
Non bacteriuric women were excluded. Inclusion criteria were not clearly outlined with little restriction on exclusion criteria. Exclusion criteria were also not clearly described. |
These were followed up weekly or biweekly for bacterial colony count to prove that the infection had cleared until delivery. |
|||||
84 treated |
|||||||
95 placebo |
|||||||
Kazemier et al |
2015 |
Randomized controlled trial embedded in a Prospective cohort. |
4283 pregnant women in screening cohort, 40 positive for asymptomatic bacteriuria were randomly assigned to treatment with nitrofurantoin, 45 positive for asymptomatic bacteriuria on placebo and remaining positive 163 were followed without treatment |
Low risk women with singleton pregnancy between 16 and 22 gestation. The women were |
Nitrofurantoin treatment/ identical placebo tablets / |
At 8 hospitals and ultrasound centres in the Netherlands. Recruitment stretched from |
A computer generated list was used. Group assignment was done on a ratio of 1:1. |
Asymptomatic for bacteriuria and gave consent to participation. |
No treatment at all |
||||||
|
|
|
|
Excluded were symptomatic women for bacteriuria, history of preterm birth before 34 weeks, foetal malformations, diabetess mellitus, and antibiotic use in the past 2 weeks, glucose 6 dehydrogenous phosphate 6 deficiencies, hypersensitivity to nitrofurantoin and risk factors for urinary tract infection. |
|
|
|
Table 1 Study characteristics
Risk of bias within studies
Methodological features for each study included in this review were assessed for risk of bias using a standard approach. A narrative summary describing risk of bias assessment is provided on Table 2.
Study |
Randomisation appropriate |
Blinding; Participant Investigator Outcome assessor |
Selective reporting |
Other factors potentially causing bias |
Intention to treat analysis appropriate |
Risk of bias at study level |
Kass 1962 |
Randomization was not clearly described. Women were randomly allocated into two study groups. One group was treated with long acting sulphonamides and the other placebo treatment. Process of randomisation was not clearly described. |
It was not clear whether participants were informed about intervention and outcome or not no details were available to clarify. |
Only bacteriuric and non bacteriuric were reported. There was no reporting of contaminated or mixed and nullified results. Several outcomes were reported on. |
Allocation concealment was not mentioned. |
Although unmentioned, Intention to treat analysis was used in this study, which was appropriate in this trial as a participants were analysed in their original group. |
Blinding was not mentioned. If it was not done, it could have introduced selection bias. Use of long acting sulphonamides only could have affected outcome analysis. Blinding was not mentioned. Group allocation was not also clearly explained. |
Nothing was stated to whether outcome assessor knew about the different study groups or did the analysis blindly. |
Identification of a case of asymptomatic bacteriruia was as judgement of significant bacteriuria was difficult. The researchers relied on clinical criteria including proteinuria and pyuria. |
|||||
Kazamier 2015 |
Randomization was proper. The inclusion of negative women in placebo group alone could have introduced bias. |
Double blinding was implemented where participant and researcher were unaware of treatment group by allocation concealment and the bacteriuria status was not revealed treatment whether placebo or nitrofurantoin was masked to participants and doctors. |
There was wide report of key findings of both primary and secondary outcomes |
Selection bias due to inclusion of women negative for ASB in the control group and were untreated but considered on analysis. The group sizes differed significantly on analysis. |
Intention to treat analysis was used and was appropriate in this trial. All participants were analysed from the group they were originally allocated. |
There was strict restriction on inclusion criteria, which may have caused a misrepresentation of population groups. Inclusion of ASB negative women in placebo and on analysis in that group. |
Table 2 Summary on risk of bias assessment
Results of individual studies
Kass 1962
Out of 84 pregnant women who were treated for ASB with long acting sulphonamides, there were 6 (7%) babies who were born preterm, compared to 26 (27%, n = 95) from the women who received a placebo. The effect size was 20%. 9 There was a benefit in reduction of premature births, of detection and treating ASB in pregnancy. The other outcomes in this study were not presented in this review.
Kazameier et al (2015)
In this study more (11 (5.3%, n = 208)) women from the group of those positive for ASB, but were untreated or given placebo had preterm birth (> 37 weeks gestation) than 2 (5%, n = 40) from women who were treated with Nitrofurantoin. 10 Only 1 (2.5%, n = 40) delivered before 34 weeks of gestation from those who were treated with Nitrofurantoin, which was less by only 1 (2 (1.0%), (OR -1.5, 95% CI, -15.3TO 18.5), for those untreated or treated with placebo. There was no statistically significant difference (OR (-0.3 (-17.2 to 16.7) in the number of women who had PB between those who were positive for asymptomatic bacteriuria and were treated with Nitrofurantoin and those who were positive but untreated or given a placebo. The risk difference was only 0.3%. Table 3 shows the results for the primary outcome. The effect size was -0.2%.
|
Deliveries |
Preterm births <37 weeks Frequency % |
Effect of Intervention OR(95% CI) |
Kass 1962 |
|
|
|
Placebo |
95 |
26 27 |
|
Treated |
84 |
6 7 |
Not provided |
Non bacteriuric |
1000 |
88 9 |
|
Kazemier 2015 |
|
|
|
Placebo/untreated |
208 |
11 5.3 |
|
Treated |
40 |
2 5.0 |
-0.3 (-17.2 to 16.7) |
Non- bacteriuric |
4035 |
207 5.1 |
|
Table 3 The preterm birth between study groups
There were conflicting results from the two trials available which evaluated effectiveness of screening and treatment of ASB in reducing PB. One trial done reported a significant reduction in risk for preterm birth following screening and treatment of ASB.9 There was a marked decrease in the number of babies delivered preterm following the treatment of ASB noted from the study. This meant that treatment of ASB was effective in reducing PB. It also meant that an infection in the urinary tract could be responsible in alterations in normal functioning of the uterus or other organs which were not directly infected. This study was done more than 5 decades ago. On the contrary there was no statistical difference in risk for delivery of preterm baby between women who were screened and treated for ASB and those who got no actual treatment but a placebo or were untreated.11 This meant that there was no adequate evidence to support screening and treatment for ASB in pregnancy. It also meant that screening and treating pregnant women for ASB was of no significant effect to the reduction of PB. A systematic review also reported that there was low quality evidence on reduced PB from treatment of ASB.12,13 Therefore there was no convincing evidence of an association between untreated ASB and PB. The two studies were both done at hospital set up, but in different nations, in America and Netherlands where prevalence and incidence of PB differed. This could have a bearing on outcome analysis. Whilst several studies report association between ASB and PB,14,6there seem to be no consensus on the effect of screening and treating the disease in reduction of PB from the randomised controlled trials that were done. Meanwhile a systematic review on several trials on effectiveness of antibiotic treatment of ASB in pregnancy have recommended it as it was associated with reduced risk of PB.15 Detection and treatment of ASB was effective in elimination of bacteriuria in pregnancy, a certain group of women were likely to have PB.9
Study limitations
There also was a There were however methodological challenges with both trials, which could have affected the analysis of the outcome. In the study11 randomization was done on a 1:1 ratio but some women who were negative for the disease were randomized into the placebo group in the process of masking the participant to treatment. There was also a huge difference in group sizes between the study groups at analysis, with 40 for treatment and 208 control groups, which could have affected outcome analysis. The trial 11 was stopped early with a smaller sample size recruited than minimum required giving adequate power value. This study also only included low risk pregnant women, which means exclusion of several other existing subgroups among the pregnant women. There were more smokers in control group than in the intervention group, which could have affected results on PB as smoking in pregnancy has been reported to be associated with PB16 However it was also argued that Missing outcome data for 5% of participants in this study could also have affected outcome results. Another limitation was that only Nitrofurantoin was used for treatment of asymptomatic bacteriruia, when drug sensitivity differs among uropathogens and especially with the increasing multidrug antibiotic resistance.17 Whilst it was stated that there was high sensitivity by the drug, there were also some resistant strains. In this era there is an increased report of rising antibiotic resistance, which calls for frequent antibiotic susceptibility tests for isolated uropathogens to use when empirically selecting antibiotics to treat the infection. There was a challenge with Kass study, where quantitation of isolated uropathogen was not clearly understood. There was a difference in the dates the trials were conducted where the oldest was conducted more than 5 decades back whilst the other was conducted 3 years back. Several analysis methods have been identified and applied in recent trials, which were not applied in this study. Applicability of the results in this era is with many doubts due to the long period before the other was done. There was more restriction on exclusion and inclusion criteria with11 trial whilst that by10 was unrestricted much, which could have caused the differences in conclusion to effectiveness of the intervention.
Recommendations
More large clinical trials are required from different settings to evaluate effectiveness of screening and treatment of asymptomatic bacteriuria to provide adequate and convincing evidence to recommend or not the screening and treatment of pregnant women for ASB as a strategy in reducing PB.
In this review pregnant women were the study population and intervention including screening and treatment of ASB was considered. The primary outcome considered for this review was PB. There were two trials which met the eligibility criteria for this review. The review done revealed that there is currently conflicting results to the effectiveness of screening and treatment of ASB in reducing PB. There is no consensus on screening and treatment for ASB in pregnancy due to lacking understanding of the effectiveness of the approach in reducing pregnancy complications. Although antibiotic treatment has been effective in clearing the infection, there still lacks universal agreement on a recommendation to screen and treat ASB. However, there was evidence that ASB play a role in prematurity. More randomized trials are needed to provide convincing evidence to recommend or not recommend screening and treatment of ASB in pregnancy.
The conduction of this review was successful through support from NORHED grant.
We would like to acknowledge the Department of Nursing Science staff for support and assistance. We also wish to acknowledge the Norway for the funding opportunity.
All authors declare that there is no conflict of interest.

©2018 Rukweza. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.